Page 534 - Elements of Chemical Reaction Engineering Ebook
P. 534
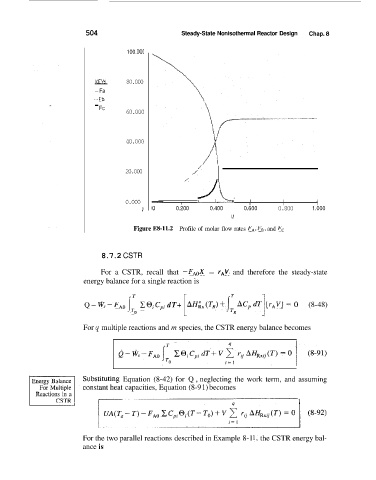
504 Steady-State Nonisothermal Reactor Design Chap. 8
100. OOC
KEY:
- 80.000
-Fa
...Fb
\
F
- C
60.000
40.000
/
20.000 ,./’
,,.’
0.000 - I I i
I
I
I
[ 10 0.200 0.400 0.600 0.800 1.000
U
Figure ES-11.2 Profile of molar flow rates FA, FB, and F,
8.7.2 CSTR
For a CSTR, recall that -FAOX = r,V and therefore the steady-state
energy balance for a single reaction is
T
Q- WT- FA, / CO,C,, dT+ [rAV] = 0 (8-48)
TO
For q multiple reactions and m species, the CSTR energy balance becomes
Equation (8-42) for Q , neglecting the work term, and assuming
capacities, Equation (8-9 1) becomes
Reactions in a
1
I
For the two parallel reactions described in Example 8-1 1, the CSTR energy bal-
ance is