Page 537 - Elements of Chemical Reaction Engineering Ebook
P. 537
Chap. 8 Summary 507
kl k2
A - 0 - C
1.000 __
1
0.500 -
250.000 350.000 450.000 550.000 , 650.000 7501.00 0
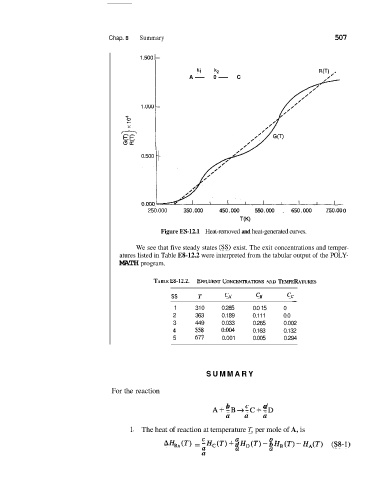
Figure ES-12.1 Heat-removed and heat-generated curves.
We see that five steady states (SS) exist. The exit concentrations and ternper-
atures listed in Table E8-12.2 were interpreted from the tabular output of the POLY-
MATH program.
TABLE E8-12.2. EFFLUENT CONCENTFCA~ONS AND TEMPERATURES
ss T CA CB CC
~ ~~
1 310 0.285 0.0 15 0
2 363 0.189 0.111 0.0
3 449 0.033 0.265 0.002
4 558 0.004 0.163 0.132
5 67i 0.001 0.005 0.294
SUMMARY
For the reaction
b d
A+-B&c+-D
a a a
1 The heat of reaction at temperature T, per mole of A, is
I
AH,,(T) = CHc(T)+,HD(T)-,~~(T)-H,(T) (S8-1)
b
d
a