Page 539 - Elements of Chemical Reaction Engineering Ebook
P. 539
Chap. 8 Summary 509
7. The CSTR energy balance is
UA
-- (T, - T) - X[Aff;,(TR] + ACp(T- TR)] = C O,Z',,(T - T,,,) (S8-9)
FA0
1. The temperature dependence of the specific reaction rate is given in
the form
(58- IO)
91. The temperature dependence of the. equilibrium constant is given by
van't Eloff's equation:
d 1nK AHRx(T)
-p =
dT RT2
If ACp = 0,
Kp(T) = Kp(T,) exp (S8-11)
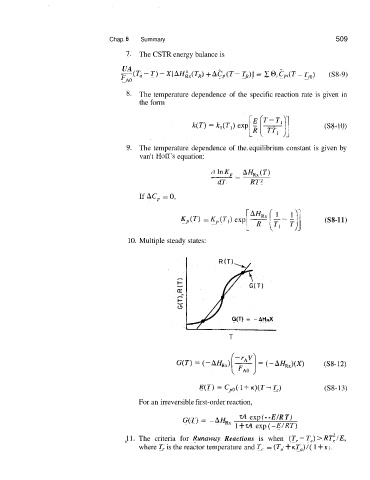
10. Multiple steady states:
G(T) = -AHRX
T
R(T) = Cpo( 1 + K)(T- T,) (S8- 13)
For an irreversible first-order reaction,
G( T) = - AHRx vl exp (- -E/ RT)
1 + 7-4 exp (- E/RT)
' 1;l. The criteria for Runaway Reactions is when (Tr- T,)> ReIE,
where ;f, is the reactor temperature and T, = (To + KT,)/( 1 + K ).