Page 554 - Elements of Chemical Reaction Engineering Ebook
P. 554
524 Steady-State Nonisothermal Reactor Design Chap. 8
(b) Let k, be the effective thermal conductivity in both the axial and radial
directions and use Fourier’s law,
dT
q =-k - dT
z e az and qr = -k, -
dr
to show that
m
82T k dT
k, -- + k, E+ - - u 1 CiC . E+ ra(Ci, T) AH,, = 0 (P8-24.2)
2
dz2 dr2 r dr 1=1 pi a2
(e) Explain the use of the following boundary conditions:
dT
At r = R, then U(T,(z) - T,) = -k, - (P8-24.3)
ar
aT
Atr=O,then - =0 (P8-24.4)
dr
(d) At the entrance and exit of the reactor, there are different boundary condi-
tions that can be used depending on the degree of sophistication required.
Explain why the simplest (see Chapter 14) set is
At z. = 0, T = To and C, = C,, (P8-24.5)
(P8-24.6)
One can usually neglect conduction in the axial directions with respect to
convection.
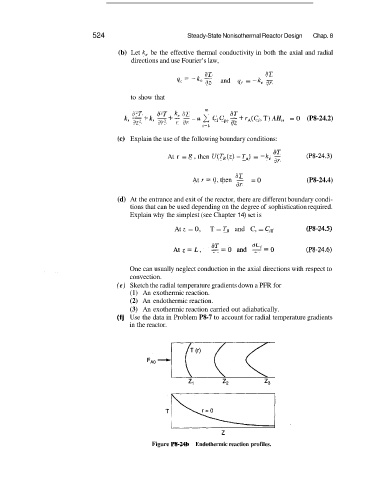
(e) Sketch the radial temperature gradients down a PFR for
(1) An exothermic reaction.
(2) An endothermic reaction.
(3) An exothermic reaction carried out adiabatically.
(fj Use the data in Problem P8-7 to account for radial temperature gradients
in the reactor.
z
Figure P8-24b Endothermic reaction profiles.