Page 555 - Elements of Chemical Reaction Engineering Ebook
P. 555
Chap. (3 Questions and Problems 525
Additional information:
cal
k, = 0.4
cm . min . K .
Reactor diameter = 3 cm
Plat temperature reaction rate as a function of radius at specific locations
down the reactor. Plot the centerline temperature as a function of conversion.
(8) What do you think about neglecting radial variations in concentration?
Read Chapter 11, then show that
m
a2T k 3T
a2T
dT
k,--fk,-+~---~ 2 CC -
3zz ar2 r ar pJ az
where De is the effective diffusivity. (Experts only)
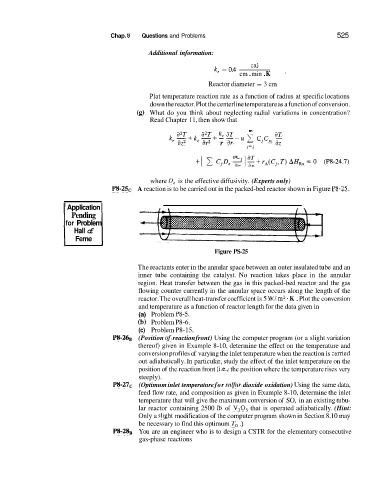
P8-2SC A reaction is to be carried out in the packed-bed reactor shown in Figure P8-25.
I Feme 1
Application
Pending
for Problem
Hall of
Figure PS-25
The reactants enter in the annular space between an outer insulated tube and an
inner tube containing the catalyst. No reaction takes place in the annular
region. Heat transfer between the gas in this packed-bed reactor and the gas
flowing counter currently in the annular space occurs along the length of the
reactor. The overall heat-transfer coefficient is 5 W/ m2 K . Plot the conversion
and temperature as a function of reactor length for the data given in
(a) Problem P8-5.
(b) Problem P8-6.
(c) Problem P8-15.
3?8-26B (Position of reaction front) Using the computer program (or a slight variation
thereof) given in Example 8-10, determine the effect on the temperature and
conversion profiles of varying the inlet temperature when the reaction is c'anied
out adiabatically. In particular, study the effect of the inlet temperature on the
position of the reaction front (i.e., the position where the temperature rises very
steeply).
PS-27, (Optimum inlet temperature for sulfur dioxide oxidation) Using the same data,
feed flow rate, and composition as given in Example 8-10, determine the inlet
temperature that will give the maximum conversion of SO, in an existing tubu-
lar reactor containing 2500 Ib of V20, that is operated adiabatically. (Hint:
Only a slight modification of the computer program shown in Section 8.10 may
be necessary to find this optimum To .)
P8-2SB You are an engineer who is to design a CSTR for the elementary consecutive
gas-phase reactions