Page 552 - Elements of Chemical Reaction Engineering Ebook
P. 552
522 Steady-State Nonisothermal Reactor Design Chap. 8
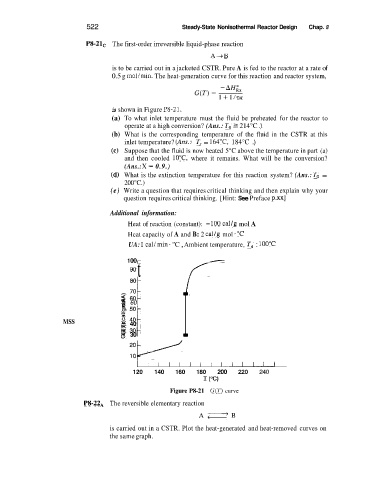
P8-2lC The first-order irreversible liquid-phase reaction
A-+B
is to be carried out in a jacketed CSTR. Pure A is fed to the reactor at a rate of
0.5 g mol/min. The heat-generation curve for this reaction and reactor system,
is shown in Figure P8-21.
(a) To what inlet temperature must the fluid be preheated for the reactor to
operate at a high conversion? (Ans.: To 2 214°C .)
(b) What is the corresponding temperature of the fluid in the CSTR at this
inlet temperature? (Am: T, = 164"C, 184°C .)
(e) Suppose that the fluid is now heated 5°C above the temperature in part (a)
and then cooled 10°C, where it remains. What will be the conversion?
(Ans.: X = 0.9.)
(a) What is the extinction temperature for this reaction system? (Ans.: To =
200°C.)
(e) Write a question that requires critical thinking and then explain why your
question requires critical thinking. [Hint: See Preface p.xx]
Additional information:
Heat of reaction (constant): - 100 cal/g mol A
Heat capacity of A and B: 2 cal/g mol "C
-
UA: 1 cal/ min - "C , Ambient temperature, T, : 100°C
1 00 ,- fl
90 t
I- '.i 30 I
60
MSS -
2 40
2ot /
.
l l l l l i l l l l l l l
120 140 160 180 200 220 240
T ("C)
Figure P8-21 G(T) curve
P8-22* The reversible elementary reaction
A ~ B
is carried out in a CSTR. Plot the heat-generated and heat-removed curves on
the same graph.