Page 168 - Academic Press Encyclopedia of Physical Science and Technology 3rd Chemical Engineering
P. 168
P1: LLL Final
Encyclopedia of Physical Science and Technology EN003H-565 June 13, 2001 20:37
230 Coherent Control of Chemical Reactions
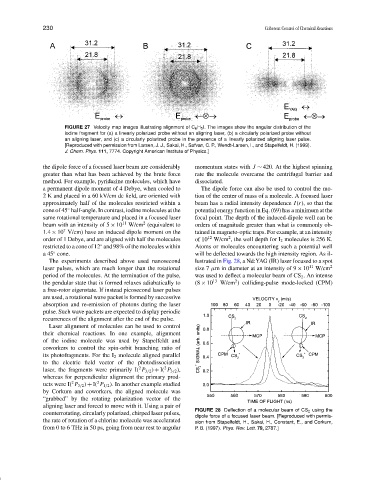
FIGURE 27 Velocity map images illustrating alignment of C 6 H 5 I. The images show the angular distribution of the
iodine fragment for (a) a linearly polarized probe without an aligning laser, (b) a circularly polarized probe without
an aligning laser, and (c) a circularly polarized probe in the presence of a linearly polarized aligning laser pulse.
[Reproduced with permission from Larsen, J. J., Sakai, H., Safvan, C. P., Wendt-Larsen, I., and Stapelfeldt, H. (1999).
J. Chem. Phys. 111, 7774. Copyright American Institute of Physics.]
the dipole force of a focused laser beam are considerably momentum states with J ∼ 420. At the highest spinning
greater than what has been achieved by the brute force rate the molecule overcame the centrifugal barrier and
method. For example, pyridazine molecules, which have dissociated.
a permanent dipole moment of 4 Debye, when cooled to The dipole force can also be used to control the mo-
2 K and placed in a 60 kV/cm dc field, are oriented with tion of the center of mass of a molecule. A focused laser
approximately half of the molecules restricted within a beam has a radial intensity dependence I(r), so that the
◦
cone of 45 half-angle. In contrast, iodine molecules at the potential energy function in Eq. (69) has a minimum at the
same rotational temperature and placed in a focused laser focal point. The depth of the induced-dipole well can be
2
beam with an intensity of 5 × 10 11 W/cm (equivalent to orders of magnitude greater than what is commonly ob-
7
1.4 × 10 V/cm) have an induced dipole moment on the tained in magneto-optic traps. For example, at an intensity
2
order of 1 Debye, and are aligned with half the molecules of 10 12 W/cm , the well depth for I 2 molecules is 256 K.
restricted to a cone of 12 and 98% of the molecules within Atoms or molecules encountering such a potential well
◦
◦
a45 cone. will be deflected towards the high intensity region. As il-
The experiments described above used nanosecond lustrated in Fig. 28, a Nd:YAG (IR) laser focused to a spot
laser pulses, which are much longer than the rotational size 7 µm in diameter at an intensity of 9 × 10 11 W/cm 2
period of the molecules. At the termination of the pulse, was used to deflect a molecular beam of CS 2 . An intense
2
the pendular state that is formed relaxes adiabatically to (8 × 10 13 W/cm ) colliding-pulse mode-locked (CPM)
a free-rotor eigenstate. If instead picosecond laser pulses
are used, a rotational wave packet is formed by successive
absorption and re-emission of photons during the laser
pulse. Such wave packets are expected to display periodic
recurrences of the alignment after the end of the pulse.
Laser alignment of molecules can be used to control
their chemical reactions. In one example, alignment
of the iodine molecule was used by Stapelfeldt and
coworkers to control the spin-orbit branching ratio of
its photofragments. For the I 2 molecule aligned parallel
to the electric field vector of the photodissociation
2
2
laser, the fragments were primarily I( P 3/2 ) + I( P 3/2 ),
whereas for perpendicular alignment the primary prod-
2
2
ucts were I( P 3/2 ) + I( P 1/2 ). In another example studied
by Corkum and coworkers, the aligned molecule was
“grabbed” by the rotating polarization vector of the
aligning laser and forced to move with it. Using a pair of
FIGURE 28 Deflection of a molecular beam of CS 2 using the
counterrotating, circularly polarized, chirped laser pulses,
dipole force of a focused laser beam. [Reproduced with permis-
the rate of rotation of a chlorine molecule was accelerated sion from Stapelfeldt, H., Sakai, H., Constant, E., and Corkum,
from 0 to 6 THz in 50 ps, going from near rest to angular P. B. (1997). Phys. Rev. Lett. 79, 2787.]