Page 205 - Academic Press Encyclopedia of Physical Science and Technology 3rd Chemical Engineering
P. 205
P1: GAE/LSK P2: FLV Final Pages
Encyclopedia of Physical Science and Technology EN004D-ID159 June 8, 2001 15:47
102 Crystallization Processes
Furthermore, the solution of a differential population bal-
ance requires that the relationship between growth rate
and size of the growing crystals be known. When all crys-
tals in the magma grow at a constant and identical rate, the
crystal–solvent system is said to follow the McCabe L
law, while systems that do not are said to exhibit anoma-
lous growth.
Two theories have been used to explain growth-rate
anomalies: size-dependent growth and growth-rate disper-
sion. As with systems that follow the L law, anomalous
growth by crystals in a multicrystal magma produces crys-
tal populations with characteristic forms. Unfortunately,
it is difficult to determine the growth mechanism from
an analysis of these forms. This means that either size-
dependent growth or growth-rate dispersion may be used
tocorrelatepopulationdensitydatawithoutacertaintythat
the correct source of anomalous growth has been identi-
fied. Determining the actual source of anomalous growth
is not trivial, but it may be worthwhile since alignment
between a mathematical model and system behavior en-
hances the utility of the model.
Size-dependent crystal growth results when the rate of FIGURE 6 Transient population density plot showing growth-rate
growth depends on the size of the growing crystal. Cer- dispersion.
tainly, this may be the case if bulk transport is the control-
lingresistancetocrystalgrowth,andtheliteratureabounds
with expressions for the appropriate mass-transfer coef- IV. PURITY, MORPHOLOGY, AND
ficients. In the more common situation in which surface SIZE DISTRIBUTIONS
integration controls growth rate, there are no mechanis-
tic relationships between growth rate and crystal size, Crystalpropertiescanbedividedintotwocategories:those
and simple empirical expressions are called upon for that basedontheindividualcrystalandthoseinvolvingallcrys-
purpose. tals of a given population. The three characteristics of the
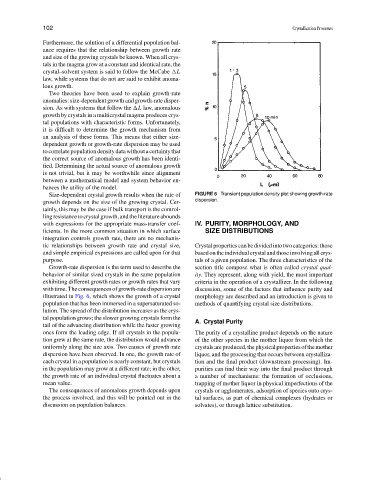
Growth-rate dispersion is the term used to describe the section title compose what is often called crystal qual-
behavior of similar sized crystals in the same population ity. They represent, along with yield, the most important
exhibiting different growth rates or growth rates that vary criteria in the operation of a crystallizer. In the following
with time. The consequences of growth-rate dispersion are discussion, some of the factors that influence purity and
illustrated in Fig. 6, which shows the growth of a crystal morphology are described and an introduction is given to
population that has been immersed in a supersaturated so- methods of quantifying crystal size distributions.
lution. The spread of the distribution increases as the crys-
tal population grows; the slower growing crystals form the
A. Crystal Purity
tail of the advancing distribution while the faster growing
ones form the leading edge. If all crystals in the popula- The purity of a crystalline product depends on the nature
tion grew at the same rate, the distribution would advance of the other species in the mother liquor from which the
uniformly along the size axis. Two causes of growth-rate crystalsareproduced,thephysicalpropertiesofthemother
dispersion have been observed. In one, the growth rate of liquor, and the processing that occurs between crystalliza-
each crystal in a population is nearly constant, but crystals tion and the final product (downstream processing). Im-
in the population may grow at a different rate; in the other, purities can find their way into the final product through
the growth rate of an individual crystal fluctuates about a a number of mechanisms: the formation of occlusions,
mean value. trapping of mother liquor in physical imperfections of the
The consequences of anomalous growth depends upon crystals or agglomerates, adsorption of species onto crys-
the process involved, and this will be pointed out in the tal surfaces, as part of chemical complexes (hydrates or
discussion on population balances. solvates), or through lattice substitution.