Page 406 - Academic Press Encyclopedia of Physical Science and Technology 3rd Chemical Engineering
P. 406
P1: GLQ Final Pages
Encyclopedia of Physical Science and Technology EN009K-419 July 19, 2001 20:57
Membranes, Synthetic, Applications 341
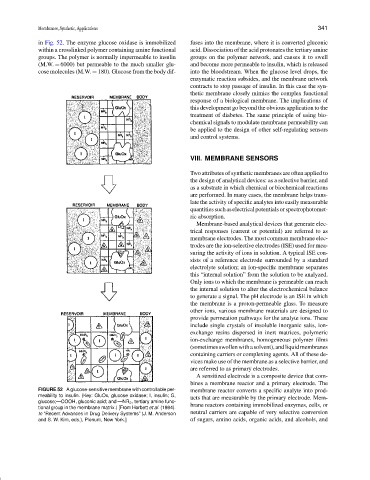
in Fig. 52. The enzyme glucose oxidase is immobilized fuses into the membrane, where it is converted gluconic
within a crosslinked polymer containing amine functional acid. Dissociation of the acid protonates the tertiary amine
groups. The polymer is normally impermeable to insulin groups on the polymer network, and causes it to swell
(M.W. = 6000) but permeable to the much smaller glu- and become more permeable to insulin, which is released
cose molecules (M.W. = 180). Glucose from the body dif- into the bloodstream. When the glucose level drops, the
enzymatic reaction subsides, and the membrane network
contracts to stop passage of insulin. In this case the syn-
thetic membrane closely mimics the complex functional
response of a biological membrane. The implications of
this development go beyond the obvious application to the
treatment of diabetes. The same principle of using bio-
chemical signals to modulate membrane permeability can
be applied to the design of other self-regulating sensors
and control systems.
VIII. MEMBRANE SENSORS
Two attributes of synthetic membranes are often applied to
the design of analytical devices: as a selective barrier, and
as a substrate in which chemical or biochemical reactions
are performed. In many cases, the membrane helps trans-
late the activity of specific analytes into easily measurable
quantitiessuchaselectricalpotentialsorspectrophotomet-
ric absorption.
Membrane-based analytical devices that generate elec-
trical responses (current or potential) are referred to as
membrane electrodes. The most common membrane elec-
trodes are the ion-selective electrodes (ISE) used for mea-
suring the activity of ions in solution. A typical ISE con-
sists of a reference electrode surrounded by a standard
electrolyte solution; an ion-specific membrane separates
this “internal solution” from the solution to be analyzed.
Only ions to which the membrane is permeable can reach
the internal solution to alter the electrochemical balance
to generate a signal. The pH electrode is an ISE in which
the membrane is a proton-permeable glass. To measure
other ions, various membrane materials are designed to
provide permeation pathways for the analyte ions. These
include single crystals of insoluble inorganic salts, ion-
exchange resins dispersed in inert matrices, polymeric
ion-exchange membranes, homogeneous polymer films
(sometimesswollenwithasolvent),andliquidmembranes
containing carriers or complexing agents. All of these de-
vices make use of the membrane as a selective barrier, and
are referred to as primary electrodes.
A sensitized electrode is a composite device that com-
bines a membrane reactor and a primary electrode. The
FIGURE 52 A glucose-sensitive membrane with controllable per- membrane reactor converts a specific analyte into prod-
meability to insulin. (Key: GluOx, glucose oxidase; I, insulin; G, ucts that are measurable by the primary electrode. Mem-
glucose;—COOH, gluconic acid; and —NR 2 , tertiary amine func-
tional group in the membrane matrix.) [From Horbett et al. (1984). brane reactors containing immobilized enzymes, cells, or
In “Recent Advances in Drug Delivery Systems” (J. M. Anderson neutral carriers are capable of very selective conversion
and S. W. Kim, eds.), Plenum, New York.] of sugars, amino acids, organic acids, and alcohols, and