Page 448 - Academic Press Encyclopedia of Physical Science and Technology 3rd Chemical Engineering
P. 448
P1: GPB/GAM P2: GQT Final Pages
Encyclopedia of Physical Science and Technology EN013A-619 July 26, 2001 19:32
Pulp and Paper 253
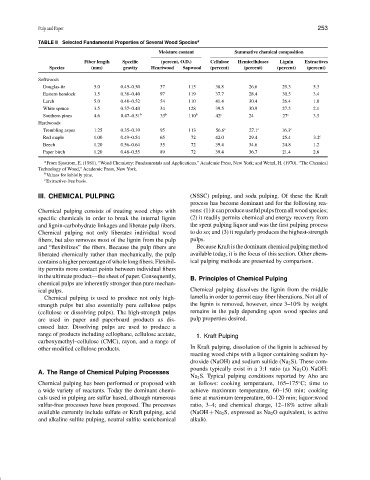
TABLE II Selected Fundamental Properties of Several Wood Species a
Moisture content Summative chemical composition
Fiber length Specific (percent, O.D.) Cellulose Hemicelluloses Lignin Extractives
Species (mm) gravity Heartwood Sapwood (percent) (percent) (percent) (percent)
Softwoods
Douglas-fir 5.0 0.45–0.50 37 115 38.8 26.6 29.3 5.3
Eastern hemlock 3.5 0.38–0.40 97 119 37.7 28.4 30.5 3.4
Larch 5.0 0.48–0.52 54 110 41.4 30.4 26.4 1.8
White spruce 3.5 0.37–0.40 34 128 39.5 30.9 27.5 2.1
Southern pines 4.6 0.47–0.51 b 33 b 110 b 42 c 24 27 c 3.5
Hardwoods
Trembling aspen 1.25 0.35–0.39 95 113 56.6 c 27.1 c 16.3 c
Red maple 1.00 0.49–0.54 65 72 42.0 29.4 25.4 3.2 c
Beech 1.20 0.56–0.64 55 72 39.4 34.6 24.8 1.2
Paper birch 1.20 0.48–0.55 89 72 39.4 36.7 21.4 2.6
a
From Sjostrom, E. (1981). “Wood Chemistry: Fundamentals and Applications.” Academic Press, New York; and Wenzl, H. (1970). “The Chemical
Technology of Wood,” Academic Press, New York.
b
Values for loblolly pine.
c
Extractive-free basis.
III. CHEMICAL PULPING (NSSC) pulping, and soda pulping. Of these the Kraft
process has become dominant and for the following rea-
Chemical pulping consists of treating wood chips with sons:(1)itcanproduceusefulpulpsfromallwoodspecies;
specific chemicals in order to break the internal lignin (2) it readily permits chemical and energy recovery from
and lignin-carbohydrate linkages and liberate pulp fibers. the spent pulping liquor and was the first pulping process
Chemical pulping not only liberates individual wood to do so; and (3) it regularly produces the highest-strength
fibers, but also removes most of the lignin from the pulp pulps.
and “flexibilizes” the fibers. Because the pulp fibers are BecauseKraftisthedominantchemicalpulpingmethod
liberated chemically rather than mechanically, the pulp available today, it is the focus of this section. Other chem-
containsahigherpercentageofwholelongfibers.Flexibil- ical pulping methods are presented by comparison.
ity permits more contact points between individual fibers
in the ultimate product—the sheet of paper. Consequently,
B. Principles of Chemical Pulping
chemical pulps are inherently stronger than pure mechan-
ical pulps. Chemical pulping dissolves the lignin from the middle
Chemical pulping is used to produce not only high- lamella in order to permit easy fiber liberations. Not all of
strength pulps but also essentially pure cellulose pulps the lignin is removed, however, since 3–10% by weight
(cellulose or dissolving pulps). The high-strength pulps remains in the pulp depending upon wood species and
are used in paper and paperboard products as dis- pulp properties desired.
cussed later. Dissolving pulps are used to produce a
range of products including cellophane, cellulose acetate,
1. Kraft Pulping
carboxymethyl–cellulose (CMC), rayon, and a range of
other modified cellulose products. In Kraft pulping, dissolution of the lignin is achieved by
reacting wood chips with a liquor containing sodium hy-
droxide (NaOH) and sodium sulfide (Na 2 S). These com-
pounds typically exist in a 3:1 ratio (as Na 2 O) NaOH:
A. The Range of Chemical Pulping Processes
Na 2 S. Typical pulping conditions reported by Aho are
Chemical pulping has been performed or proposed with as follows: cooking temperature, 165–175 C; time to
◦
a wide variety of reactants. Today the dominant chemi- achieve maximum temperature, 60–150 min; cooking
cals used in pulping are sulfur based, although numerous time at maximum temperature, 60–120 min; liquor:wood
sulfur-free processes have been proposed. The processes ratio, 3–4; and chemical charge, 12–18% active alkali
available currently include sulfate or Kraft pulping, acid (NaOH + Na 2 S, expressed as Na 2 O equivalent, is active
and alkaline sulfite pulping, neutral sulfite semichemical alkali).