Page 170 - Academic Press Encyclopedia of Physical Science and Technology 3rd Organic Chemistry
P. 170
P1: GOL Final Pages
Encyclopedia of Physical Science and Technology EN007F-314 July 6, 2001 16:59
326 Heterocyclic Chemistry
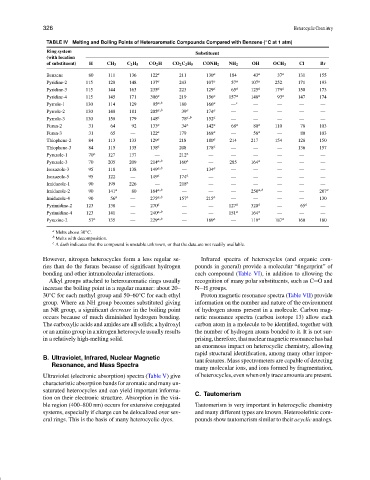
TABLE IV Melting and Boiling Points of Heteroaromatic Compounds Compared with Benzene ( C at 1 atm)
◦
Ring system
Substituent
(with location
of substituent) H CH 3 C 2 H 5 CO 2 H CO 2 C 2 H 5 CONH 2 NH 2 OH OCH 3 Cl Br
Benzene 80 111 136 122 a 211 130 a 184 43 a 37 a 131 155
Pyridine-2 115 128 148 137 a 243 107 a 57 a 107 a 252 171 193
Pyridine-3 115 144 163 235 a 223 129 a 65 a 125 a 179 a 150 173
Pyridine-4 115 145 171 306 a 219 156 a 157 a 148 a 93 a 147 174
Pyrrole-1 130 114 129 95 a,b 180 166 a — c — — — —
Pyrrole-2 130 148 181 205 a,b 39 a 174 a — — — — —
Pyrrole-3 130 158 179 148 a 78 a,b 152 a — — — — —
Furan-2 31 64 92 133 a 34 a 142 a 68 a 80 a 110 78 103
Furan-3 31 65 — 122 a 179 168 a — 58 a — 80 103
Thiophene-2 84 113 133 129 a 218 180 a 214 217 154 128 150
Thiophene-3 84 115 135 138 a 208 178 a — — — 136 157
Pyrazole-1 70 a 127 137 — 212 a — — — — — —
Pyrazole-3 70 205 209 214 a,b 160 a — 285 164 a — — —
Isoxazole-3 95 118 138 149 a,b — 134 a — — — — —
Isoxazole-5 95 122 — 149 a 174 a — — — — — —
Imidazole-1 90 199 226 — 218 a — — — — — —
Imidazole-2 90 141 a 80 164 a,b — — — 250 a,b — — 207 a
Imidazole-4 90 56 a — 275 a,b 157 a 215 a — — — — 130
Pyrimidine-2 123 138 — 270 a — — 127 a 320 a — 65 a —
Pyrimidine-4 123 141 — 240 a,b — — 151 a 164 a — — —
Pyrazine-2 57 a 135 — 229 a,b — 189 a — 119 a 187 a 160 180
a Melts above 30 C.
◦
b Melts with decomposition.
c A dash indicates that the compound is unstable unknown, or that the data are not readily available.
However, nitrogen heterocycles form a less regular se- Infrared spectra of heterocycles (and organic com-
ries than do the furans because of significant hydrogen pounds in general) provide a molecular “fingerprint” of
bonding and other intramolecular interactions. each compound (Table VI), in addition to allowing the
Alkyl groups attached to heteroaromatic rings usually recognition of many polar substituents, such as C O and
increase the boiling point in a regular manner: about 20– N H groups.
30 C for each methyl group and 50–60 C for each ethyl Proton magnetic resonance spectra (Table VII) provide
◦
◦
group. Where an NH group becomes substituted giving information on the number and nature of the environment
an NR group, a significant decrease in the boiling point of hydrogen atoms present in a molecule. Carbon mag-
occurs because of much diminished hydrogen bonding. netic resonance spectra (carbon isotope 13) allow each
The carboxylic acids and amides are all solids; a hydroxyl carbon atom in a molecule to be identified, together with
or an amino group in a nitrogen heterocycle usually results the number of hydrogen atoms bonded to it. It is not sur-
in a relatively high-melting solid. prising,therefore,thatnuclearmagneticresonancehashad
an enormous impact on heterocyclic chemistry, allowing
rapid structural identification, among many other impor-
B. Ultraviolet, Infrared, Nuclear Magnetic tant features. Mass spectrometers are capable of detecting
Resonance, and Mass Spectra
many molecular ions, and ions formed by fragmentation,
Ultraviolet (electronic absorption) spectra (Table V) give of heterocycles, even when only trace amounts are present.
characteristic absorption bands for aromatic and many un-
saturated heterocycles and can yield important informa-
C. Tautomerism
tion on their electronic structure. Absorption in the visi-
ble region (400–800 nm) occurs for extensive conjugated Tautomerism is very important in heterocyclic chemistry
systems, especially if charge can be delocalized over sev- and many different types are known. Heteroolefinic com-
eral rings. This is the basis of many heterocyclic dyes. pounds show tautomerism similar to their acyclic analogs.