Page 171 - Academic Press Encyclopedia of Physical Science and Technology 3rd Organic Chemistry
P. 171
P1: GOL Final Pages
Encyclopedia of Physical Science and Technology EN007F-314 July 6, 2001 16:59
Heterocyclic Chemistry 327
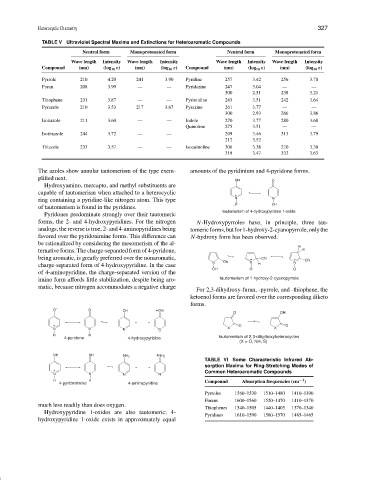
TABLE V Ultraviolet Spectral Maxima and Extinctions for Heteroaromatic Compounds
Neutral form Monoprotonated form Neutral form Monoprotonated form
Wave length Intensity Wave length Intensity Wave length Intensity Wave length Intensity
Compound (nm) (log 10 ε) (nm) (log 10 ε) Compound (nm) (log 10 ε) (nm) (log 10 ε)
Pyrrole 210 4.20 241 3.90 Pyridine 257 3.42 256 3.70
Furan 208 3.90 — — Pyridazine 247 3.04 — —
300 2.51 238 3.21
Thiophene 231 3.87 — — Pyrimidine 243 3.51 242 3.64
Pyrazole 210 3.53 217 3.67 Pyrazine 261 3.77 — —
300 2.93 266 3.86
Isoxazole 211 3.60 — — Indole 270 3.77 280 3.68
Quinoline 275 3.51 — —
Isothiazole 244 3.72 — — 299 3.46 313 3.79
212 3.52 — —
Thiazole 233 3.57 — — Isoquinoline 306 3.38 270 3.30
319 3.47 332 3.63
The azoles show annular tautomerism of the type exem- amounts of the pyridinium and 4-pyridone forms.
plified next. OH O
Hydroxyamino, mercapto, and methyl substituents are
capable of tautomerism when attached to a heterocyclic
+
ring containing a pyridine-like nitrogen atom. This type N N
_ O OH
of tautomerism is found in the pyridines.
tautomerism of 4-hydroxypyridine 1-oxide
Pyridones predominate strongly over their tautomeric
forms, the 2- and 4-hydroxypyridines. For the nitrogen N-Hydroxypyrroles have, in principle, three tau-
analogs, the reverse is true, 2- and 4-aminopyridines being tomeric forms, but for 1-hydroxy-2-cyanopyrrole, only the
favored over the pyridonimine forms. This difference can N-hydroxy form has been observed.
be rationalized by considering the mesomerism of the al-
H
ternative forms. The charge-separated form of 4-pyridone, H
being aromatic, is greatly preferred over the nonaromatic, + CN + CN
charge-separated form of 4-hydroxypyridine. In the case N CN N H N
OH _ O _ O
of 4-aminopyridine, the charge-separated version of the
imino form affords little stabilization, despite being aro- tautomerism of 1-hydroxy-2-cyanopyrrole
matic, because nitrogen accommodates a negative charge
For 2,3-dihydroxy-furan, -pyrrole, and -thiophene, the
ketoenol forms are favored over the corresponding diketo
forms.
_
O O OH + OH
O OH
+ O O
N N N N _ X X
H H tautomerism of 2,3-dihydroxyheterocycles
4-pyridone 4-hydroxypyridine
(X = O, NH, S)
_
NH NH NH 2 + NH 2
TABLE VI Some Characteristic Infrared Ab-
sorption Maxima for Ring-Stretching Modes of
+ Common Heteroaromatic Compounds
N N N N _
H H Compound Absorption frequencies (cm −1 )
4-pyridonimine 4-aminopyridine
Pyrroles 1560–1530 1510–1480 1410–1390
Furans 1600–1560 1520–1470 1410–1370
much less readily than does oxygen.
Thiophenes 1540–1505 1440–1405 1370–1340
Hydroxypyridine 1-oxides are also tautomeric; 4-
Pyridines 1610–1590 1580–1570 1485–1465
hydroxypyridine 1-oxide exists in approximately equal