Page 208 - Academic Press Encyclopedia of Physical Science and Technology 3rd Organic Chemistry
P. 208
P1: LLL/GVX P2: GQT Final Pages
Encyclopedia of Physical Science and Technology EN011G-539 July 14, 2001 21:48
Organic Chemical Systems, Theory 455
largest for small cycles and decrease as the number of
AOs in the cycle increases. It has become customary to
refer to the pericyclic reaction paths proceeding through
aromatic transition states as “allowed” and to those pro-
ceeding through antiaromatic transition states as “forbid-
den.” Pericyclic reactions of both types can occur, but the
former are normally strongly favored if all other factors
are the same. Many different theoretical approaches, of
which we mention only two here, have been used to derive
simple rules that enable one to predict whether a particu-
lar pericyclic process will be of the allowed or forbidden
kind. The rules have become known as the Woodward–
Hoffmann rules, although many other workers have also
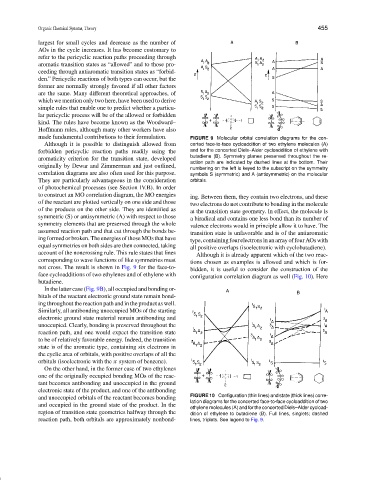
made fundamental contributions to their formulation. FIGURE 9 Molecular orbital correlation diagrams for the con-
Although it is possible to distinguish allowed from certed face-to-face cycloaddition of two ethylene molecules (A)
forbidden pericyclic reaction paths readily using the and for the concerted Diels–Alder cycloaddition of ethylene with
butadiene (B). Symmetry planes preserved throughout the re-
aromaticity criterion for the transition state, developed
action path are indicated by dashed lines at the bottom. Their
originally by Dewar and Zimmerman and just outlined, numbering on the left is keyed to the subscript on the symmetry
correlation diagrams are also often used for this purpose. symbols S (symmetric) and A (antisymmetric) on the molecular
They are particularly advantageous in the consideration orbitals.
of photochemical processes (see Section IV.B). In order
to construct an MO correlation diagram, the MO energies
ing. Between them, they contain two electrons, and these
of the reactant are plotted vertically on one side and those
two electrons do not contribute to bonding in the molecule
of the products on the other side. They are identified as
at the transition state geometry. In effect, the molecule is
symmetric (S) or antisymmetric (A) with respect to those
a biradical and contains one less bond than its number of
symmetry elements that are preserved through the whole
valence electrons would in principle allow it to have. The
assumed reaction path and that cut through the bonds be-
transition state is unfavorable and is of the antiaromatic
ing formed or broken. The energies of those MOs that have
type, containing fourelectrons in an array of fourAOs with
equal symmetries on both sides are then connected, taking all positive overlaps (isoelectronic with cyclobutadiene).
account of the noncrossing rule. This rule states that lines Although it is already apparent which of the two reac-
corresponding to wave functions of like symmetries must tions chosen as examples is allowed and which is for-
not cross. The result is shown in Fig. 9 for the face-to- bidden, it is useful to consider the construction of the
face cycloadditions of two ethylenes and of ethylene with configuration correlation diagram as well (Fig. 10). Here
butadiene.
In the latter case (Fig. 9B), all occupied and bonding or-
bitals of the reactant electronic ground state remain bond-
ing throughout the reaction path and in the product as well.
Similarly, all antibonding unoccupied MOs of the starting
electronic ground state material remain antibonding and
unoccupied. Clearly, bonding is preserved throughout the
reaction path, and one would expect the transition state
to be of relatively favorable energy. Indeed, the transition
state is of the aromatic type, containing six electrons in
the cyclic area of orbitals, with positive overlaps of all the
orbitals (isoelectronic with the π system of benzene).
On the other hand, in the former case of two ethylenes
one of the originally occupied bonding MOs of the reac-
tant becomes antibonding and unoccupied in the ground
electronic state of the product, and one of the antibonding
and unoccupied orbitals of the reactant becomes bonding FIGURE 10 Configuration (thin lines) and state (thick lines) corre-
lation diagrams for the concerted face-to-face cycloaddition of two
and occupied in the ground state of the product. In the
ethylene molecules (A) and for the concerted Diels–Alder cycload-
region of transition state geometries halfway through the dition of ethylene to butadiene (B). Full lines, singlets; dashed
reaction path, both orbitals are approximately nonbond- lines, triplets. See legend to Fig. 9.