Page 246 - Academic Press Encyclopedia of Physical Science and Technology 3rd Organic Chemistry
P. 246
P1: GJC/GLE P2: GNB Final Pages
Encyclopedia of Physical Science and Technology EN011A-544 July 25, 2001 18:30
Organometallic Chemistry 533
n
The reaction is much faster if the metal has a d config- organic compound so as to carry out a reaction that would
uration between 2 and 10, because d-electrons stabilize not otherwise take place.
the transition state (or most unstable species along the
LCl 2 Pt(C 2 H 4 ) + Me 2 NH
reaction pathway; the more stable this is, the faster the
−
reaction). = [LCl 2 PtC 2 H 4 NMe 2 ] + H + (12)
6
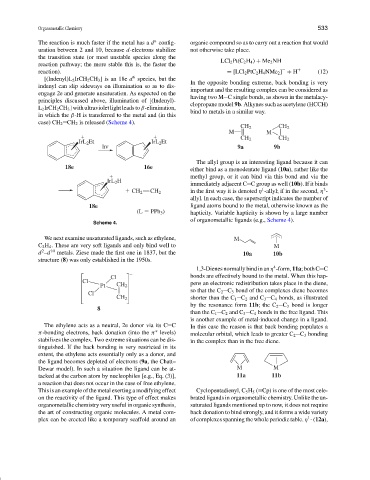
[(Indenyl)L 2 IrCH 2 CH 3 ] is an 18e d species, but the
In the opposite bonding extreme, back bonding is very
indenyl can slip sideways on illumination so as to dis-
important and the resulting complex can be considered as
engage 2e and generate unsaturation. As expected on the
having two M C single bonds, as shown in the metalacy-
principles discussed above, illumination of [(Indenyl)-
clopropane model 9b. Alkynes such as acetylene (HCCH)
L 2 IrCH 2 CH 3 ] with ultraviolet light leads to β-elimination,
bind to metals in a similar way.
in which the β-H is transferred to the metal and (in this
case) CH 2 CH 2 is released (Scheme 4).
CH 2 CH 2
M M
CH 2 CH 2
IrL 2 Et IrL 2 Et
hν 9a 9b
The allyl group is an interesting ligand because it can
18e 16e either bind as a monodentate ligand (10a), rather like the
methyl group, or it can bind via this bond and via the
IrL 2 H
immediately adjacent C C group as well (10b). If it binds
1
3
in the first way it is denoted η -allyl; if in the second, η -
CH 2 CH 2
allyl. In each case, the superscript indicates the number of
18e ligand atoms bound to the metal, otherwise known as the
(L PPh 3 ) hapticity. Variable hapticity is shown by a large number
of organometallic ligands (e.g., Scheme 4).
Scheme 4.
We next examine unsaturated ligands, such as ethylene, M
C 2 H 4 . These are very soft ligands and only bind well to M
2
d –d 10 metals. Ziese made the first one in 1837, but the 10a 10b
structure (8) was only established in the 1950s.
4
1,3-Dienes normally bind in an η -form, 11a; both C C
Cl bonds are effectively bound to the metal. When this hap-
Cl pens an electronic redistribution takes place in the diene,
Pt CH 2
so that the C 2 C 3 bond of the complexes diene becomes
Cl
CH 2 shorter than the C 1 C 2 and C 3 C 4 bonds, as illustrated
by the resonance form 11b; the C 2 C 3 bond is longer
8
than the C 1 C 2 and C 3 C 4 bonds in the free ligand. This
is another example of metal-induced change in a ligand.
The ethylene acts as a neutral, 2e donor via its C C In this case the reason is that back bonding populates a
∗
π-bonding electrons, back donation (into the π levels) molecular orbital, which leads to greater C 2 C 3 bonding
stabilizes the complex. Two extreme situations can be dis- in the complex than in the free diene.
tinguished. If the back bonding is very restricted in its
extent, the ethylene acts essentially only as a donor, and
the ligand becomes depleted of electrons (9a, the Chatt–
Dewar model). In such a situation the ligand can be at- M M
tacked at the carbon atom by nucleophiles [e.g., Eq. (3)], 11a 11b
a reaction that does not occur in the case of free ethylene.
This is an example of the metal exerting a modifying effect Cyclopentadienyl, C 5 H 5 ( Cp) is one of the most cele-
on the reactivity of the ligand. This type of effect makes brated ligands in organometallic chemistry. Unlike the un-
organometallic chemistry very useful in organic synthesis, saturated ligands mentioned up to now, it does not require
the art of constructing organic molecules. A metal com- back donation to bind strongly, and it forms a wide variety
1
plex can be erected like a temporary scaffold around an of complexes spanning the whole periodic table. η -(12a),