Page 273 - Academic Press Encyclopedia of Physical Science and Technology 3rd Organic Chemistry
P. 273
P1: LDK/GJK P2: GQT/Final Pages
Encyclopedia of Physical Science and Technology EN012G-576 July 28, 2001 12:44
Physical Organic Chemistry 219
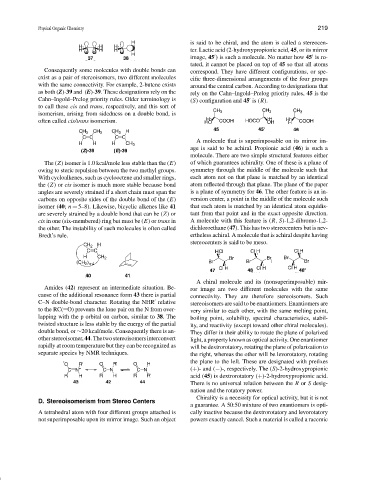
is said to be chiral, and the atom is called a stereocen-
ter. Lactic acid (2-hydroxypropionic acid, 45, or its mirror
image, 45 ) is such a molecule. No matter how 45 is ro-
tated, it cannot be placed on top of 45 so that all atoms
Consequently some molecules with double bonds can correspond. They have different configurations, or spe-
exist as a pair of stereoisomers, two different molecules cific three-dimensional arrangements of the four groups
with the same connectivity. For example, 2-butene exists around the central carbon. According to designations that
as both (Z)-39 and (E)-39. These designations rely on the rely on the Cahn–Ingold–Prelog priority rules, 45 is the
Cahn–Ingold–Prelog priority rules. Older terminology is (S) configuration and 45 is (R).
to call these cis and trans, respectively, and this sort of
isomerism, arising from sidedness on a double bond, is
often called cis/trans isomerism.
A molecule that is superimposable on its mirror im-
age is said to be achiral. Propionic acid (46) is such a
molecule. There are two simple structural features either
The (Z) isomer is 1.0 kcal/mole less stable than the (E) of which guarantees achirality. One of these is a plane of
owing to steric repulsion between the two methyl groups. symmetry through the middle of the molecule such that
With cycloalkenes, such as cyclooctene and smaller rings, each atom not on that plane is matched by an identical
the (Z)or cis isomer is much more stable because bond atom reflected through that plane. The plane of the paper
angles are severely strained if a short chain must span the is a plane of symmetry for 46. The other feature is an in-
carbons on opposite sides of the double bond of the (E) version center, a point in the middle of the molecule such
isomer (40; n = 5–8). Likewise, bicyclic alkenes like 41 that each atom is matched by an identical atom equidis-
are severely strained by a double bond that can be (Z)or tant from that point and in the exact opposite direction.
cis in one (six-membered) ring but must be (E)or trans in A molecule with this feature is (R, S)-1,2-dibromo-1,2-
the other. The instability of such molecules is often called dichloroethane (47). This has two stereocenters but is nev-
Bredt’s rule. ertheless achiral. A molecule that is achiral despite having
stereocenters is said to be meso.
A chiral molecule and its (nonsuperimposable) mir-
Amides (42) represent an intermediate situation. Be- ror image are two different molecules with the same
cause of the additional resonance form 43 there is partial connectivity. They are therefore stereoisomers. Such
C–N double-bond character. Rotating the NHR relative stereoisomers are said to be enantiomers. Enantiomers are
to the RC( O) prevents the lone pair on the N from over- very similar to each other, with the same melting point,
lapping with the p orbital on carbon, similar to 38. The boiling point, solubility, spectral characteristics, stabil-
twisted structure is less stable by the energy of the partial ity, and reactivity (except toward other chiral molecules).
double bond, or ∼20 kcal/mole. Consequently there is an- They differ in their ability to rotate the plane of polarized
otherstereoisomer,44.Thetwostereoisomersinterconvert light, a property known as optical activity. One enantiomer
rapidly at room temperature but they can be recognized as will be dextrorotatory, rotating the plane of polarization to
separate species by NMR techniques. the right, whereas the other will be levorotatory, rotating
the plane to the left. These are designated with prefixes
(+)- and (−)-, respectively. The (S)-2-hydroxypropionic
acid (45) is dextrorotatory (+)-2-hydroxypropionic acid.
There is no universal relation between the R or S desig-
nation and the rotatory power.
Chirality is a necessity for optical activity, but it is not
D. Stereoisomerism from Stereo Centers
a guarantee. A 50:50 mixture of two enantiomers is opti-
A tetrahedral atom with four different groups attached is cally inactive because the dextrorotatory and levorotatory
not superimposable upon its mirror image. Such an object powers exactly cancel. Such a material is called a racemic