Page 272 - Academic Press Encyclopedia of Physical Science and Technology 3rd Organic Chemistry
P. 272
P1: LDK/GJK P2: GQT/Final Pages
Encyclopedia of Physical Science and Technology EN012G-576 July 28, 2001 12:44
218 Physical Organic Chemistry
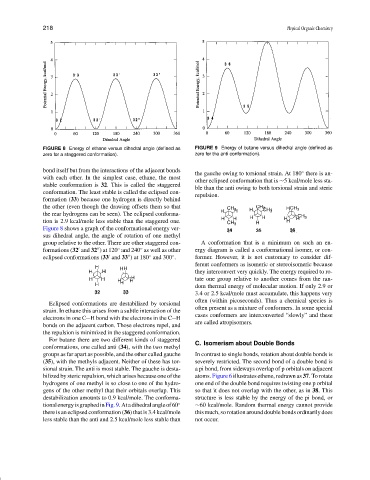
FIGURE 8 Energy of ethane versus dihedral angle (defined as FIGURE 9 Energy of butane versus dihedral angle (defined as
zero for a staggered conformation). zero for the anti conformation).
bond itself but from the interactions of the adjacent bonds ◦
the gauche owing to torsional strain. At 180 there is an-
with each other. In the simplest case, ethane, the most
other eclipsed conformation that is ∼5 kcal/mole less sta-
stable conformation is 32. This is called the staggered
ble than the anti owing to both torsional strain and steric
conformation. The least stable is called the eclipsed con-
repulsion.
formation (33) because one hydrogen is directly behind
the other (even though the drawing offsets them so that
the rear hydrogens can be seen). The eclipsed conforma-
tion is 2.9 kcal/mole less stable than the staggered one.
Figure 8 shows a graph of the conformational energy ver-
sus dihedral angle, the angle of rotation of one methyl
group relative to the other. There are other staggered con- A conformation that is a minimum on such an en-
◦ ◦
formations (32 and 32 ) at 120 and 240 as well as other ergy diagram is called a conformational isomer, or con-
◦
◦
eclipsed conformations (33 and 33 ) at 180 and 300 . former. However, it is not customary to consider dif-
ferent conformers as isomeric or stereoisomeric because
they interconvert very quickly. The energy required to ro-
tate one group relative to another comes from the ran-
dom thermal energy of molecular motion. If only 2.9 or
3.4 or 2.5 kcal/mole must accumulate, this happens very
often (within picoseconds). Thus a chemical species is
Eclipsed conformations are destabilized by torsional
often present as a mixture of conformers. In some special
strain. In ethane this arises from a subtle interaction of the
cases conformers are interconverted “slowly” and these
electrons in one C H bond with the electrons in the C H
are called atropisomers.
bonds on the adjacent carbon. Those electrons repel, and
the repulsion is minimized in the staggered conformation.
For butane there are two different kinds of staggered C. Isomerism about Double Bonds
conformations, one called anti (34), with the two methyl
groups as far apart as possible, and the other called gauche In contrast to single bonds, rotation about double bonds is
(35), with the methyls adjacent. Neither of these has tor- severely restricted. The second bond of a double bond is
sional strain. The anti is most stable. The gauche is desta- a pi bond, from sideways overlap of p orbitals on adjacent
bilized by steric repulsion, which arises because one of the atoms. Figure 6 illustrates ethene, redrawn as 37. To rotate
hydrogens of one methyl is so close to one of the hydro- one end of the double bond requires twisting one p orbital
gens of the other methyl that their orbitals overlap. This so that it does not overlap with the other, as in 38. This
destabilization amounts to 0.9 kcal/mole. The conforma- structure is less stable by the energy of the pi bond, or
tionalenergyisgraphedinFig.9.Atadihedralangleof60 ◦ ∼60 kcal/mole. Random thermal energy cannot provide
there is an eclipsed conformation (36) that is 3.4 kcal/mole this much, so rotation around double bonds ordinarily does
less stable than the anti and 2.5 kcal/mole less stable than not occur.