Page 301 - Academic Press Encyclopedia of Physical Science and Technology 3rd Organic Chemistry
P. 301
P1: GRB Final Pages
Encyclopedia of Physical Science and Technology EN016B-738 July 31, 2001 14:0
82 Stereochemistry
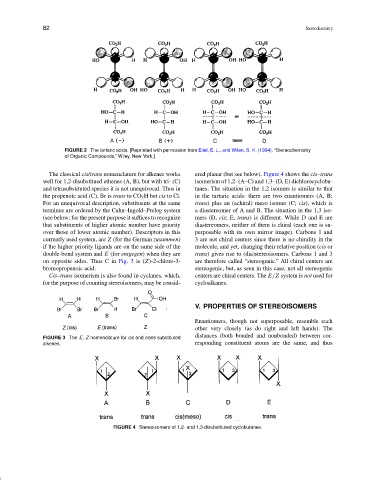
FIGURE 2 The tartaric acids. [Reprinted with permission from Eliel, E. L., and Wilen, S. H. (1994). “Stereochemistry
of Organic Compounds,” Wiley, New York.]
The classical cis/trans nomenclature for alkenes works ered planar (but see below). Figure 4 shows the cis–trans
well for 1,2-disubstitued ethenes (A, B), but with tri- (C) isomerism of 1,2- (A–C) and 1,3- (D, E) dichlorocyclobu-
and tetrasubstituted species it is not unequivocal. Thus in tanes. The situation in the 1,2 isomers is similar to that
the propenoic acid (C), Br is trans to CO 2 Hbut cis to Cl. in the tartaric acids: there are two enantiomers (A, B;
For an unequivocal description, substituents at the same trans) plus an (achiral) meso isomer (C; cis), which is
terminus are ordered by the Cahn–Ingold–Prelog system a diastereomer of A and B. The situation in the 1,3 iso-
(see below; for the present purpose it suffices to recognize mers (D, cis;E, trans) is different. While D and E are
that substituents of higher atomic number have priority diastereomers, neither of them is chiral (each one is su-
over those of lower atomic number). Descriptors in this perposable with its own mirror image). Carbons 1 and
currently used system, are Z (for the German zusammen) 3 are not chiral centers since there is no chirality in the
if the higher priority ligands are on the same side of the molecule, and yet, changing their relative position (cis or
double-bond system and E (for entgegen) when they are trans) gives rise to (dia)stereoisomers. Carbons 1 and 3
on opposite sides. Thus C in Fig. 3 is (Z)-2-chloro-3- are therefore called “stereogenic.” All chiral centers are
bromopropenoic acid. stereogenic, but, as seen in this case, not all stereogenic
Cis–trans isomerism is also found in cyclanes, which, centers are chiral centers. The E/Z system is not used for
for the purpose of counting stereoisomers, may be consid- cycloalkanes.
V. PROPERTIES OF STEREOISOMERS
Enantiomers, though not superposable, resemble each
other very closely (as do right and left hands). The
distances (both bonded and nonbonded) between cor-
FIGURE 3 The E, Z nomenclature for cis and trans substituted
alkenes. responding constituent atoms are the same, and thus
FIGURE 4 Stereoisomers of 1,2- and 1,3-disubstituted cyclobutanes.