Page 296 - Academic Press Encyclopedia of Physical Science and Technology 3rd Organic Chemistry
P. 296
P1: LDK/GJK P2: GQT/Final Pages
Encyclopedia of Physical Science and Technology EN012G-576 July 28, 2001 12:44
242 Physical Organic Chemistry
selection for thermodynamic favorability. If α is near 1, quirements. The chlorination reaction is rather unselec-
then the thermodynamic stabilities fully influence the acti- tive, consistent with a very stable product, owing to the
vation energy. Thus α is a quantitative measure of product- strength of the H Cl bond that is formed (BDE = 103
development control, or the extent to which the transition kcal/mole,comparedto87kcal/moleforH Br).Theother
state partakes of the stability of the product. In a very ap- reactions are more selective, especially with Cl 3 C·. Fur-
proximate sense, α represents a measure of the extent to thermore, the selectivity with NBS is the same, within
which the transition state resembles the product. For ex- experimental error, as with Br·. It would be very fortu-
ample, in the above hydrolysis of ethyl vinyl ether, the α itous for the N-bromosuccinimidyl radical to have the
of 0.68 can be identified loosely with the partial negative same selectivity as Br·. Therefore it was concluded that
charge δ− acquired by A in the transition state (178). the reactive radical in NBS brominations is Br· and not
N-bromosuccinimidyl.
δ+ δ-
C 2 H 5 O C C HA ‡
R H + X·→ R·+ H X. (54)
H H 2
178
If instead the product becomes more and more stable,
C. Hammett Equation
the product curve in Fig. 19 would drop, and its inter-
section with the reactant curve would also drop in en- There is no requirement that the G and G in Eq. (53)
‡
◦
ergy. Thus the activation energy is lowered, opposite to must refer to rates and equilibria of the same reaction. The
the change above, where the product became less stable. following Hammett equation is an extension to the com-
Moreover, as the product curve drops, its intersection with parison of substituent effects in two different reactions:
the reactant curve moves back (to the left) along the re-
log k X = ρσ X + c. (55)
action coordinate. Thus the transition state resembles the 10
reactant more and more. There is less and less product- Here the substituent constant σ X is defined by the fol-
development control, and α diminishes. In the extreme lowing equation from the measured acid-dissociation con-
case of a very stable product the reaction becomes fast but stants of substituted and unsubstituted benzoic acids, and
unselective. k X and k H are rate constants for substituted and unsubsti-
Some of these conclusions can be seen in the reac- tuted reactants:
tions of some hydrocarbons with radical species. The rel-
XC 6 H 4 COOH C 6 H 5 COOH
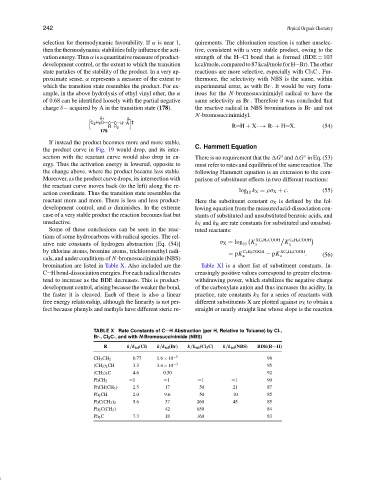
ative rate constants of hydrogen abstraction [Eq. (54)] σ X = log 10 K a K a
by chlorine atoms, bromine atoms, trichloromethyl radi- C 6 H 5 COOH XC 6 H 4 COOH
= pK a − pK a . (56)
cals, and under conditions of N-bromosuccinimide (NBS)
bromination are listed in Table X. Also included are the Table XI is a short list of substituent constants. In-
C Hbond-dissociationenergies.Foreachradicaltherates creasingly positive values correspond to greater electron-
tend to increase as the BDE decreases. This is product- withdrawing power, which stabilizes the negative charge
development control, arising because the weaker the bond, of the carboxylate anion and thus increases the acidity. In
the faster it is cleaved. Each of these is also a linear practice, rate constants k X for a series of reactants with
free energy relationship, although the linearity is not per- different substituents X are plotted against σ X to obtain a
fect because phenyls and methyls have different steric re- straight or nearly straight line whose slope is the reaction
TABLE X Rate Constants of C H Abstraction (per H, Relative to Toluene) by Cl·,
Br·, Cl 3 C·, and with N-Bromosuccinimide (NBS)
R k /k tol (Cl) k /k tol (Br) k /k tol (Cl 3 C) k /k tol (NBS) BDE(R H)
CH 3 CH 2 0.77 1.6 × 10 −5 98
(CH 3 ) 2 CH 3.3 3.4 × 10 −3 95
(CH 3 ) 3 C 4.6 0.30 92
PhCH 2 =1 =1 =1 =1 90
PhCH(CH 3 ) 2.5 17 50 21 87
Ph 2 CH 2.0 9.6 50 10 85
PhC(CH 3 ) 2 5.6 37 260 45 85
Ph 2 C(CH 3 ) 42 650 84
Ph 3 C 7.3 18 160 83