Page 292 - Academic Press Encyclopedia of Physical Science and Technology 3rd Organic Chemistry
P. 292
P1: LDK/GJK P2: GQT/Final Pages
Encyclopedia of Physical Science and Technology EN012G-576 July 28, 2001 12:44
238 Physical Organic Chemistry
the requirement for overlap between the positive lobe of antiaromatic, with four electrons delocalized. Therefore
one orbital and the negative lobe of another. Such an ar- the former reaction is quite facile, but the latter does not
rangement of orbitals has the topology of a M¨obius strip, occur thermally. It does occur photochemically, with ap-
obtained from a rectangular ribbon by twisting one end propriate substituents to facilitate creation of the excited
◦
by 180 before fastening it to the other end. The normal electronic state. Alternatively, a cycloaddition with 4n de-
topology, often called H¨uckel in this context, always has localized electrons can occur if the orbital overlaps can
positive lobes overlapping positive (or negative overlap- achieve a M¨obius topology. This requires addition across
ping negative). According to molecular orbital calcula- opposite faces of a pi systems. Such an addition is called
tions, stabilization and aromaticity can be associated with antarafacial, in contrast to the usual suprafacial, across a
all M¨obius cycles containing 4n delocalized electrons, and single face of a pi system. Usually this latter is sterically
destabilization and antiaromaticity with 4n + 2. the only possibility. However, an alkene can add across
a ketene R 2 C C O via transition state 145, with over-
lap between the positive lobe of an alkene carbon and the
negative lobe of the carbonyl carbon.
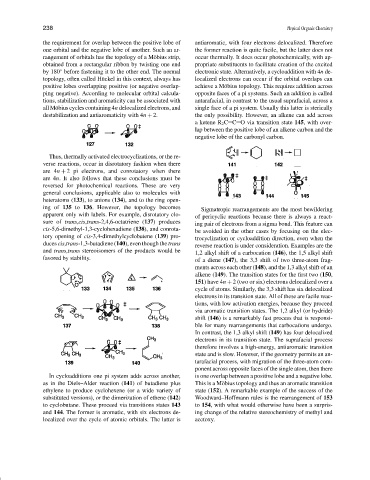
Thus, thermally activated electrocyclizations, or the re-
verse reactions, occur in disrotatory fashion when there
are 4n + 2 pi electrons, and conrotatory when there
are 4n. It also follows that these conclusions must be
reversed for photochemical reactions. These are very
general conclusions, applicable also to molecules with
heteratoms (133), to anions (134), and to the ring open-
ing of 135 to 136. However, the topology becomes Sigmatropic rearrangements are the most bewildering
apparent only with labels. For example, disrotatory clo- of pericyclic reactions because there is always a react-
sure of trans,cis,trans-2,4,6-octatriene (137) produces ing pair of electrons from a sigma bond. This feature can
cis-5,6-dimethyl-1,3-cyclohexadiene (138), and conrota- be avoided in the other cases by focusing on the elec-
tory opening of cis-3,4-dimethylcyclobutene (139) pro- trocyclization or cycloaddition direction, even when the
duces cis,trans-1,3-butadiene (140), even though the trans reverse reaction is under consideration. Examples are the
and trans,trans stereoisomers of the products would be 1,2 alkyl shift of a carbocation (146), the 1,5 alkyl shift
favored by stability. of a diene (147), the 3,3 shift of two three-atom frag-
ments across each other (148), and the 1,3 alkyl shift of an
alkene (149). The transition states for the first two (150,
151)have4n + 2 (two or six) electrons delocalized over a
cycle of atoms. Similarly, the 3,3 shift has six delocalized
electrons in its transition state. All of these are facile reac-
tions, with low activation energies, because they proceed
via aromatic transition states. The 1,2 alkyl (or hydride)
shift (146) is a remarkably fast process that is responsi-
ble for many rearrangements that carbocations undergo.
In contrast, the 1,3 alkyl shift (149) has four delocalized
electrons in its transition state. The suprafacial process
therefore involves a high-energy, antiaromatic transition
state and is slow. However, if the geometry permits an an-
tarafacial process, with migration of the three-atom com-
ponent across opposite faces of the single atom, then there
In cycloadditions one pi system adds across another, is one overlap between a positive lobe and a negative lobe.
as in the Diels–Alder reaction (141) of butadiene plus This is a M¨obius topology and thus an aromatic transition
ethylene to produce cyclohexene (or a wide variety of state (152). A remarkable example of the success of the
substituted versions), or the dimerization of ethene (142) Woodward–Hoffmann rules is the rearrangement of 153
to cyclobutane. These proceed via transitions states 143 to 154, with what would otherwise have been a surpris-
and 144. The former is aromatic, with six electrons de- ing change of the relative stereochemistry of methyl and
localized over the cycle of atomic orbitals. The latter is acetoxy.