Page 288 - Academic Press Encyclopedia of Physical Science and Technology 3rd Organic Chemistry
P. 288
P1: LDK/GJK P2: GQT/Final Pages
Encyclopedia of Physical Science and Technology EN012G-576 July 28, 2001 12:44
234 Physical Organic Chemistry
18
(2R,3R)-3-phenyl-2-butyl acetate (110), with retention acetate in alkaline H 2 O produces acetate containing the
of configuration. Even more remarkable is that (2R,3S)- 18 O and n-pentanol without any 18 O:
3-phenyl-2-butyl tosylate (111) reacts in acetic acid to
18 −
form equal amounts of (2R,3S)- and (2S,3R)-3-phenyl- CH 3 C( O)OC 5 H 11 + OH
2-butyl acetate (112 + 112 ). Again the reaction proceeds → CH 3 C( O) O + C 5 H 11 OH. (44)
18
−
with retention of configuration, but here the product is
racemic. These are reaction conditions that would fa- This experiment could also be performed with
18
vor as rate-limiting step the formation of a carbocation, CH 3 C( O) OC 5 H 11 to verify that the 18 O C pentyl
PhCH(CH 3 )CH CH 3 , rather than attack by acetate, as bond does not cleave. However, it is easier to “label” that
+
16
18
with 107. However, that carbocation would be the same position with ordinary O and run the reaction in H 2 O.
from either 109 or 111, which should then have given A rare exception to this behavior is with the highly hind-
the same products. Instead, to avoid so unstable an inter- ered methyl 2,4,6-tri-tert-butylbenzoate, where hydro-
18
18
mediate, the pi electrons of the phenyl group serve as an lysis in H 2 O produces CH 3 OH by alkyl–oxygen
internal nucleophile. As in 108, the configuration at C2 cleavage.
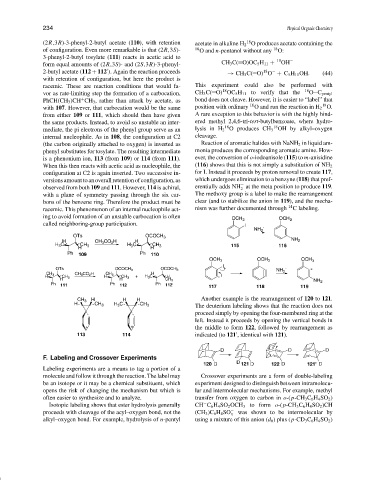
(the carbon originally attached to oxygen) is inverted as Reaction of aromatic halides with NaNH 2 in liquid am-
phenyl substitutes for tosylate. The resulting intermediate monia produces the corresponding aromatic amine. How-
is a phenonium ion, 113 (from 109)or 114 (from 111). ever, the conversion of o-iodoanisole (115)to m-anisidine
When this then reacts with acetic acid as nucleophile, the (116) shows that this is not simply a substitution of NH 2
configuration at C2 is again inverted. Two successive in- for I. Instead it proceeds by proton removal to create 117,
versions amount to an overall retention of configuration, as which undergoes elimination to a benzyne (118) that pref-
−
observed from both 109 and 111. However, 114 is achiral, erentially adds NH at the meta position to produce 119.
2
with a plane of symmetry passing through the six car- The methoxy group is a label to make the rearrangement
bons of the benzene ring. Therefore the product must be clear (and to stabilize the anion in 119), and the mecha-
racemic. This phenomenon of an internal nucleophile act- nism was further documented through 14 C labeling.
ing to avoid formation of an unstable carbocation is often
called neighboring-group participation.
Another example is the rearrangement of 120 to 121.
The deuterium labeling shows that the reaction does not
proceed simply by opening the four-membered ring at the
left. Instead it proceeds by opening the vertical bonds in
the middle to form 122, followed by rearrangement as
indicated (to 121 , identical with 121).
F. Labeling and Crossover Experiments
Labeling experiments are a means to tag a portion of a
molecule and follow it through the reaction. The label may Crossover experiments are a form of double-labeling
be an isotope or it may be a chemical substituent, which experiment designed to distinguish between intramolecu-
opens the risk of changing the mechanism but which is lar and intermolecular mechanisms. For example, methyl
often easier to synthesize and to analyze. transfer from oxygen to carbon in o-(p-CH 3 C 6 H 4 SO 2 )
Isotopic labeling shows that ester hydrolysis generally CH C 6 H 4 SO 2 OCH 3 to form o-(p-CH 3 C 6 H 4 SO 2 )CH
−
proceeds with cleavage of the acyl–oxygen bond, not the (CH 3 )C 6 H 4 SO − was shown to be intermolecular by
3
alkyl–oxygen bond. For example, hydrolysis of n-pentyl using a mixture of this anion (d 0 ) plus (p-CD 3 C 6 H 4 SO 2 )