Page 293 - Academic Press Encyclopedia of Physical Science and Technology 3rd Organic Chemistry
P. 293
P1: LDK/GJK P2: GQT/Final Pages
Encyclopedia of Physical Science and Technology EN012G-576 July 28, 2001 12:44
Physical Organic Chemistry 239
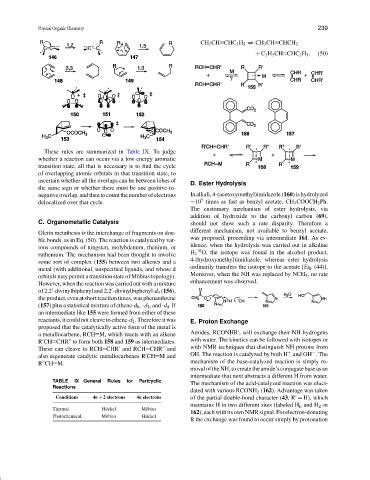
CH 3 CH CHC 2 H 5 CH 3 CH CHCH 3
+ C 2 H 5 CH CHC 2 H 5 . (50)
These rules are summarized in Table IX. To judge
whether a reaction can occur via a low-energy aromatic
transition state, all that is necessary is to find the cycle
of overlapping atomic orbitals in that transition state, to
ascertain whether all the overlaps can be between lobes of
D. Ester Hydrolysis
the same sign or whether there must be one positive-to-
negative overlap, and then to count the number of electrons In alkali, 4-(acetoxymethyl)imidazole (160) is hydrolyzed
5
delocalized over that cycle. ∼10 times as fast as benzyl acetate, CH 3 COOCH 2 Ph.
The customary mechanism of ester hydrolysis, via
addition of hydroxide to the carbonyl carbon (69),
C. Organometallic Catalysis should not show such a rate disparity. Therefore a
Olefin metathesis is the interchange of fragments on dou- different mechanism, not available to benzyl acetate,
ble bonds, as in Eq. (50). The reaction is catalyzed by var- was proposed, proceeding via intermediate 161.Asev-
ious compounds of tungsten, molybdenum, rhenium, or idence, when the hydrolysis was carried out in alkaline
18
H 2 O, the isotope was found in the alcohol product,
ruthenium. The mechanism had been thought to involve
4-(hydroxymethyl)imidazole, whereas ester hydrolysis
some sort of complex (155) between two alkenes and a
ordinarily transfers the isotope to the acetate [Eq. (44)].
metal (with additional, unspecified ligands, and whose d
Moreover, when the NH was replaced by NCH 3 , no rate
orbitals may permit a transition state of M¨obius topology).
enhancement was observed.
However, when the reaction was carried out with a mixture
of 2,2 -divinylbiphenyl and 2,2 -divinylbiphenyl-d 4 (156),
theproduct,evenatshortreactiontimes,wasphenanthrene
(157) plus a statistical mixture of ethene-d 0 , -d 2 , and -d 4 . If
an intermediate like 155 were formed from either of these
reactants, it could not cleave to ethene-d 2 . Therefore it was E. Proton Exchange
proposed that the catalytically active form of the metal is
a metallocarbene, RCH M, which reacts with an alkene Amides, RCONHR , will exchange their NH hydrogens
R CH CHR to form both 158 and 159 as intermediates. with water. The kinetics can be followed with isotopes or
These can cleave to RCH CHR and RCH CHR and with NMR techniques that distinguish NH protons from
+
−
also regenerate catalytic metallocarbenes R CH M and OH. The reaction is catalyzed by both H and OH . The
R CH M. mechanism of the base-catalyzed reaction is simply re-
moval of the NH, to create theamide’s conjugate base as an
intermediate that next abstracts a different H from water.
TABLE IX General Rules for Pericyclic The mechanism of the acid-catalyzed reaction was eluci-
Reactions
dated with various RCONH 2 (162). Advantage was taken
Conditions 4n + 2 electrons 4n electrons of the partial double-bond character (43,R = H), which
maintains H in two different sites (labeled H E and H Z in
Thermal H¨uckel M¨obius
162),eachwithitsownNMRsignal.Forelectron-donating
Photochemical M¨obius H¨uckel
R the exchange was found to occur simply by protonation