Page 289 - Academic Press Encyclopedia of Physical Science and Technology 3rd Organic Chemistry
P. 289
P1: LDK/GJK P2: GQT/Final Pages
Encyclopedia of Physical Science and Technology EN012G-576 July 28, 2001 12:44
Physical Organic Chemistry 235
−
CH C 6 H 4 SO 2 OCD 3 (d 6 ) and observing that a statistical second step is rate-limiting. The magnitude of k 1 is irrele-
mixture of d 0 , d 3 , and d 6 products was formed. The in- vant to the question of which step is rate-limiting, but if
tramolecular methyl transfer would require transition state k 1 k −1 + k 2 , then the concentration of intermediate B
◦
123 but with a 180 CH ··· CH 3 ·· · O angle, to be con- must be very small.
sistent with inversion of configuration at the methyl, and In some cases k −1 or k 2 in Eqs. (45)–(48) is not a rate
this geometry is impossible. This reasoning is known as constant but a rate coefficient, depending on the concen-
the endocyclic restriction test. tration of some other species. If so, it may be possible to
vary that concentration so that the first step is rate-limiting
under some conditions and the second step is rate-limiting
under others. The change of rate-limiting step then appears
as a characteristic dependence of rate on that concentra-
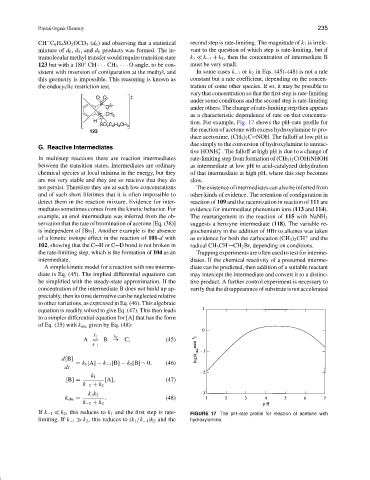
tion. For example, Fig. 17 shows the pH–rate profile for
the reaction of acetone with excess hydroxylamine to pro-
duce acetoxime, (CH 3 ) 2 C NOH. The falloff at low pH is
due simply to the conversion of hydroxylamine to unreac-
G. Reactive Intermediates
+
tive HONH . The falloff at high pH is due to a change of
3
In multistep reactions there are reaction intermediates rate-limiting step from formation of (CH 3 ) 2 C(OH)NHOH
between the transition states. Intermediates are ordinary as intermediate at low pH to acid-catalyzed dehydration
chemical species at local minima in the energy, but they of that intermediate at high pH, where this step becomes
are not very stable and they are so reactive that they do slow.
not persist. Therefore they are at such low concentrations The existence of intermediates can also be inferred from
and of such short lifetimes that it is often impossible to other kinds of evidence. The retention of configuration in
detect them in the reaction mixture. Evidence for inter- reaction of 109 and the racemization in reaction of 111 are
mediates sometimes comes from the kinetic behavior. For evidence for intermediate phenonium ions (113 and 114).
example, an enol intermediate was inferred from the ob- The rearrangement in the reaction of 115 with NaNH 2
servation that the rate of bromination of acetone [Eq. (38)] suggests a benzyne intermediate (118). The variable re-
is independent of [Br 2 ]. Another example is the absence giochemistry in the addition of HBr to alkenes was taken
of a kinetic isotope effect in the reaction of 101-d with as evidence for both the carbocation (CH 3 ) 2 CH and the
+
102, showing that the C HorC D bond is not broken in radical CH 3 CH CH 2 Br, depending on conditions.
·
the rate-limiting step, which is the formation of 104 as an Trapping experiments are often used to test for interme-
intermediate. diates. If the chemical reactivity of a presumed interme-
A simple kinetic model for a reaction with one interme- diate can be predicted, then addition of a suitable reactant
diate is Eq. (45). The implied differential equations can may intercept the intermediate and convert it to a distinc-
be simplified with the steady-state approximation. If the tive product. A further control experiment is necessary to
concentration of the intermediate B does not build up ap- verify that the disappearance of substrate is not accelerated
preciably, then its time derivative can be neglected relative
to other variations, as expressed in Eq. (46). This algebraic
equation is readily solved to give Eq. (47). This then leads
to a simpler differential equation for [A] that has the form
of Eq. (35) with k obs given by Eq. (48):
k 1 k 2
A B → C, (45)
k −1
d[B]
= k 1 [A] − k −1 [B] − k 2 [B] ∼ 0, (46)
dt
k 1
[B] = [A], (47)
k −1 + k 2
k 1 k 2
k obs = . (48)
k −1 + k 2
If k −1 k 2 , this reduces to k 1 and the first step is rate- FIGURE 17 The pH–rate profile for reaction of acetone with
limiting. If k −1 k 2 , this reduces to (k 1 /k −1 )k 2 and the hydroxylamine.