Page 287 - Academic Press Encyclopedia of Physical Science and Technology 3rd Organic Chemistry
P. 287
P1: LDK/GJK P2: GQT/Final Pages
Encyclopedia of Physical Science and Technology EN012G-576 July 28, 2001 12:44
Physical Organic Chemistry 233
Because m D > m H the zero-point energy is greater for a (CH 3 ) 2 CH stabilized by six hyperconjugative resonance
+
C H bond than for a C D, as shown in Fig. 16, and the forms. The alternative regiochemistry would proceed via
bond-dissociation energy is less for C H than for C D. theintermediateCH 3 CH 2 CH ,withonlythreesuchforms
+
2
Then, according to Fig. 12, a C H bond will break faster. (from delocalization of electrons in two C H bonds and
For typical values of k F , the rate constant ratio, k H /k D ,is oneC C).However,inthepresenceofperoxides,bromine
◦
∼7at25 C. atoms are generated and a free-radical chain process in-
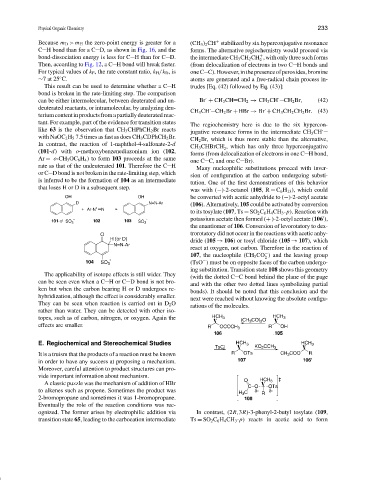
This result can be used to determine whether a C H trudes [Eq. (42) followed by Eq. (43)]:
bond is broken in the rate-limiting step. The comparison
·
·
can be either intermolecular, between deuterated and un- Br + CH 3 CH CH 2 → CH 3 CH CH 2 Br, (42)
deuterated reactants, or intramolecular, by analyzing deu-
CH 3 CH CH 2 Br +HBr → Br + CH 3 CH 2 CH 2 Br. (43)
·
·
teriumcontentinproductsfromapartiallydeuteratedreac-
tant. For example, part of the evidence for transition states
The regiochemistry here is due to the six hypercon-
like 63 is the observation that CH 3 CHPhCH 2 Br reacts ·
jugative resonance forms in the intermediate CH 3 CH
with NaOC 2 H 5 7.5 times as fast as does CH 3 CDPhCH 2 Br.
CH 2 Br, which is thus more stable than the alternative,
In contrast, the reaction of 1-naphthol-4-sulfonate-2-d ·
CH 3 CHBrCH , which has only three hyperconjugative
2
(101-d) with o-methoxybenzenediazonium ion (102,
forms (from delocalization of electrons in one C H bond,
Ar = o-CH 3 OC 6 H 4 ) to form 103 proceeds at the same
one C C, and one C Br).
rate as that of the undeuterated 101. Therefore the C H
Many nucleophilic substitutions proceed with inver-
or C D bond is not broken in the rate-limiting step, which sion of configuration at the carbon undergoing substi-
is inferred to be the formation of 104 as an intermediate tution. One of the first demonstrations of this behavior
that loses H or D in a subsequent step. was with (−)-2-octanol (105,R = C 6 H 13 ), which could
be converted with acetic anhydride to (−)-2-octyl acetate
(106). Alternatively, 105 could be activated by conversion
to its tosylate (107,Ts = SO 2 C 6 H 4 CH 3 -p). Reaction with
potassium acetate then formed (+ )-2-octyl acetate (106 ),
the enantiomer of 106. Conversion of levorotatory to dex-
trorotatory did not occur in the reactions with acetic anhy-
dride (105 → 106) or tosyl chloride (105 → 107), which
react at oxygen, not carbon. Therefore in the reaction of
107, the nucleophile (CH 3 CO ) and the leaving group
−
2
−
(TsO ) must be on opposite faces of the carbon undergo-
ing substitution. Transition state 108 shows this geometry
The applicability of isotope effects is still wider. They
(with the dotted C C bond behind the plane of the page
can be seen even when a C HorC D bond is not bro-
and with the other two dotted lines symbolizing partial
ken but when the carbon bearing H or D undergoes re-
bonds). It should be noted that this conclusion and the
hybridization, although the effect is considerably smaller.
next were reached without knowing the absolute configu-
They can be seen when reaction is carried out in D 2 O
rations of the molecules.
rather than water. They can be detected with other iso-
topes, such as of carbon, nitrogen, or oxygen. Again the
effects are smaller.
E. Regiochemical and Stereochemical Studies
It is a truism that the products of a reaction must be known
in order to have any success at proposing a mechanism.
Moreover, careful attention to product structures can pro-
vide important information about mechanism.
A classic puzzle was the mechanism of addition of HBr
to alkenes such as propene. Sometimes the product was
2-bromopropane and sometimes it was 1-bromopropane.
Eventually the role of the reaction conditions was rec-
ognized. The former arises by electrophilic addition via In contrast, (2R,3R)-3-phenyl-2-butyl tosylate (109,
transition state 65, leading to the carbocation intermediate Ts = SO 2 C 6 H 4 CH 3 -p) reacts in acetic acid to form