Page 306 - Academic Press Encyclopedia of Physical Science and Technology 3rd Organic Chemistry
P. 306
P1: GRB Final Pages
Encyclopedia of Physical Science and Technology EN016B-738 July 31, 2001 14:0
Stereochemistry 87
with another. The two chiral centers need not be in the inferred. Thanks to the availability of powerful computers,
same molecule; as will be shown later, configurational cor- it has also become increasingly feasible to derive absolute
relations between two similar molecules can sometimes configuration from optical rotation or circular dichroism
be based on comparison of their optical rotations, opti- spectra (see below) by theoretical computation. In some
cal rotatory dispersion, or circular dichroism spectra (see cases, absolute configuration can also be established by
below). It is also possible to tie two chiral centers, one examining the crystal habit (macroscopic dimensions) of
of known absolute configuration, the other unknown, to- a crystal in the presence of certain impurities (Addadi
gether chemically by either ionic or covalent bonds and et al., 1986).
determine their relative configuration by X-ray diffraction Once the absolute configurations of a few chiral
or other means. Since the absolute configuration of one of molecules are known, those of others can be established
the chiral centers is known, that of the other can then be de- by correlation.
duced. If the compound containing that center can then be
separated by an appropriate chemical reaction, its optical X. CHIRALITY IN ABSENCE
rotation can be measured and thus the necessary correla- OF CHIRAL CENTERS
tion between optical rotation ( + or − ) and configuration
(S or R) is established. [There is no general relation what- Although Le Bel’s and van’t Hoff’s understanding of chi-
ever between + and − (experimental quantities) and R rality rested on the concept of tetrahedral carbon or, more
and S (descriptors).] generally, of what is now called a chiral center, chirality
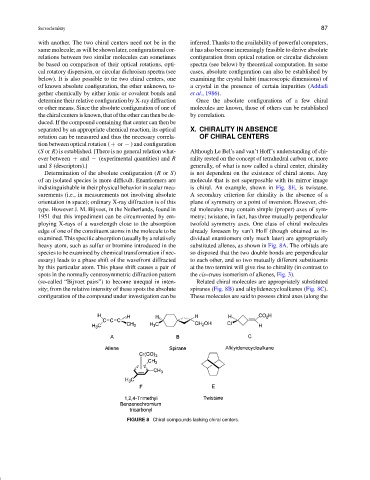
Determination of the absolute configuration (R or S) is not dependent on the existence of chiral atoms. Any
of an isolated species is more difficult. Enantiomers are molecule that is not superposable with its mirror image
indistinguishable in their physical behavior in scalar mea- is chiral. An example, shown in Fig. 8E, is twistane.
surements (i.e., in measurements not involving absolute A secondary criterion for chirality is the absence of a
orientation in space); ordinary X-ray diffraction is of this plane of symmetry or a point of inversion. However, chi-
type. However J. M. Bijvoet, in the Netherlands, found in ral molecules may contain simple (proper) axes of sym-
1951 that this impediment can be circumvented by em- metry; twistane, in fact, has three mutually perpendicular
ploying X-rays of a wavelength close to the absorption twofold symmetry axes. One class of chiral molecules
edge of one of the constituent atoms in the molecule to be already foreseen by van’t Hoff (though obtained as in-
examined. This specific absorption (usually by a relatively dividual enantiomers only much later) are appropriately
heavy atom, such as sulfur or bromine introduced in the substituted allenes, as shown in Fig. 8A. The orbitals are
species to be examined by chemical transformation if nec- so disposed that the two double bonds are perpendicular
essary) leads to a phase shift of the wavefront diffracted to each other, and so two mutually different substituents
by this particular atom. This phase shift causes a pair of at the two termini will give rise to chirality (in contrast to
spots in the normally centrosymmetric diffraction pattern the cis–trans isomerism of alkenes, Fig. 3).
(so-called “Bijvoet pairs”) to become unequal in inten- Related chiral molecules are appropriately substituted
sity; from the relative intensity of these spots the absolute spiranes (Fig. 8B) and alkylidenecycloalkanes (Fig. 8C).
configuration of the compound under investigation can be These molecules are said to possess chiral axes (along the
FIGURE 8 Chiral compounds lacking chiral centers.