Page 309 - Academic Press Encyclopedia of Physical Science and Technology 3rd Organic Chemistry
P. 309
P1: GRB Final Pages
Encyclopedia of Physical Science and Technology EN016B-738 July 31, 2001 14:0
90 Stereochemistry
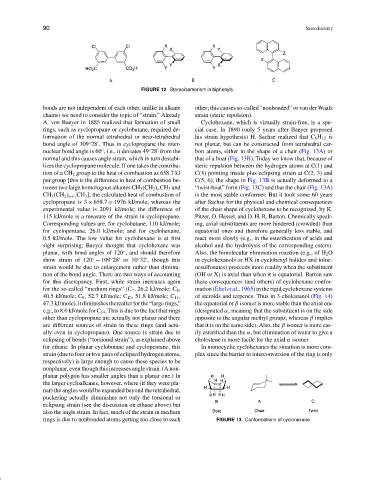
FIGURE 12 Stereoisomerism in biphenyls.
bonds are not independent of each other, unlike in alkane other; this causes so-called “nonbonded” or van der Waals
chains) we need to consider the topic of “strain.” Already strain (steric repulsion).
A. von Baeyer in 1885 realized that formation of small Cyclohexane, which is virtually strain-free, is a spe-
rings, such as cyclopropane or cyclobutane, required de- cial case. In 1890 (only 5 years after Baeyer proposed
formation of the normal tetrahedral or near-tetrahedral his strain hypothesis) H. Sachse realized that C 6 H 12 is
◦
bond angle of 109 28 . Thus in cyclopropane the inter- not planar, but can be constructed from tetrahedral car-
◦
nuclear bond angle is 60 , i.e., it deviates 49 28 from the bon atoms, either in the shape of a chair (Fig. 13A) or
◦
normal and this causes angle strain, which in turn destabi- that of a boat (Fig. 13B). Today we know that, because of
lizes the cyclopropane molecule. If one takes the contribu- steric repulsion between the hydrogen atoms at C(1) and
tion of a CH 2 group to the heat of combustion as 658.7 kJ C(4) pointing inside plus eclipsing strain at C(2, 3) and
per group [this is the difference in heat of combustion be- C(5, 6), the shape in Fig. 13B is actually deformed to a
tween two large homologous alkanes CH 3 (CH 2 ) n CH 3 and “twist-boat” form (Fig. 13C) and that the chair (Fig. 13A)
CH 3 (CH 2 ) n +1 CH 3 ], the calculated heat of combustion of is the most stable conformer. But it took some 60 years
cyclopropane is 3 × 658.7 = 1976 kJ/mole, whereas the after Sachse for the physical and chemical consequences
experimental value is 2091 kJ/mole; the difference of of the chair shape of cyclohexane to be recognized, by K.
115 kJ/mole is a measure of the strain in cyclopropane. Pitzer, O. Hassel, and D. H. R. Barton. Chemically speak-
Corresponding values are, for cyclobutane, 110 kJ/mole; ing, axial substituents are more hindered (crowded) than
for cyclopentane, 26.0 kJ/mole; and for cyclohexane, equatorial ones and therefore generally less stable, and
0.5 kJ/mole. The low value for cyclohexane is at first react more slowly (e.g., in the esterification of acids and
sight surprising; Baeyer thought that cyclohexane was alcohol and the hydrolysis of the corresponding esters).
planar, with bond angles of 120 , and should therefore Also, the bimolecular elimination reaction (e.g., of H 2 O
◦
◦
◦
show strain of 120 − 109 28 or 10 32 , though this in cyclohexanols or HX in cyclohexyl halides and tolue-
◦
strain would be due to enlargement rather than diminu- nesulfonates) proceeds more readily when the substituent
tion of the bond angle. There are two ways of accounting (OH or X) is axial than when it is equatorial. Barton saw
for this discrepancy. First, while strain increases again these consequences (and others) of cyclohexane confor-
for the so-called “medium rings” (C 7 , 26.2 kJ/mole; C 8 , mation (Eliel et al., 1965) in the rigid cyclohexane systems
40.5 kJ/mole; C 9 , 52.7 kJ/mole; C 10 , 51.8 kJ/mole; C 11 , of steroids and terpenes. Thus in 3-cholestanol (Fig. 14)
47.3kJ/mole),itdiminishesthereafterforthe“largerings,” the equatorial or β isomer is more stable than the axial one
e.g., to 8.0 kJ/mole for C 14 . This is due to the fact that rings (designated α, meaning that the substituent is on the side
other than cyclopropane are actually not planar and there opposite to the angular methyl groups, whereas β implies
are different sources of strain in these rings (and actu- that it is on the same side). Also, the β isomer is more eas-
ally even in cyclopropane). One source is strain due to ily esterified than the α, but elimination of water to give a
eclipsing of bonds (“torsional strain”), as explained above cholestene is more facile for the axial α isomer.
for ethane. In planar cyclobutane and cyclopentane, this In monocyclic cyclohexanes the situation is more com-
strain (due to four or five pairs of eclipsed hydrogen atoms, plex since the barrier to interconversion of the ring is only
respectively) is large enough to cause these species to be
nonplanar, even though this increases angle strain. (A non-
planar polygon has smaller angles than a planar one.) In
the larger cycloalkanes, however, where (if they were pla-
nar) the angles would be expanded beyond the tetrahedral,
puckering actually diminishes not only the torsional or
eclipsing strain (see the discussion on ethane above) but
also the angle strain. In fact, much of the strain in medium
rings is due to nonbonded atoms getting too close to each FIGURE 13 Conformations of cyclohexane.