Page 307 - Academic Press Encyclopedia of Physical Science and Technology 3rd Organic Chemistry
P. 307
P1: GRB Final Pages
Encyclopedia of Physical Science and Technology EN016B-738 July 31, 2001 14:0
88 Stereochemistry
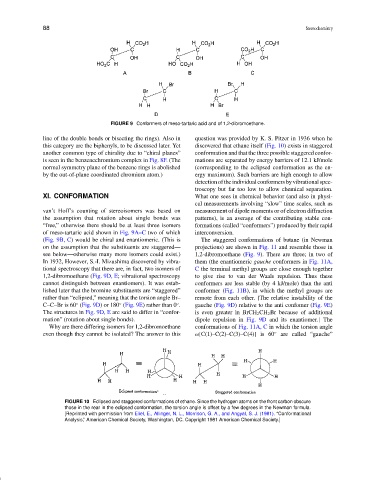
FIGURE 9 Conformers of meso-tartaric acid and of 1,2-dibromoethane.
line of the double bonds or bisecting the rings). Also in question was provided by K. S. Pitzer in 1936 when he
this category are the biphenyls, to be discussed later. Yet discovered that ethane itself (Fig. 10) exists in staggered
another common type of chirality due to “chiral planes” conformation and that the three possible staggered confor-
is seen in the benzenechromium complex in Fig. 8F. (The mations are separated by energy barriers of 12.1 kJ/mole
normal symmetry plane of the benzene rings is abolished (corresponding to the eclipsed conformation as the en-
by the out-of-plane coordinated chromium atom.) ergy maximum). Such barriers are high enough to allow
detection ofthe individual conformers by vibrationalspec-
troscopy but far too low to allow chemical separation.
XI. CONFORMATION What one sees in chemical behavior (and also in physi-
cal measurements involving “slow” time scales, such as
van’t Hoff’s counting of stereoisomers was based on measurement of dipole moments or of electron diffraction
the assumption that rotation about single bonds was patterns), is an average of the contributing stable con-
“free,” otherwise there should be at least three isomers formations (called “conformers”) produced by their rapid
of meso-tartaric acid shown in Fig. 9A–C two of which interconversion.
(Fig. 9B, C) would be chiral and enantiomeric. (This is The staggered conformations of butane (in Newman
on the assumption that the substituents are staggered— projections) are shown in Fig. 11 and resemble those in
see below—otherwise many more isomers could exist.) 1,2-dibromoethane (Fig. 9). There are three; in two of
In 1932, However, S.-I. Mizushima discovered by vibra- them (the enantiomeric gauche conformers in Fig. 11A,
tional spectroscopy that there are, in fact, two isomers of C the terminal methyl groups are close enough together
1,2-dibromoethane (Fig. 9D, E; vibrational spectroscopy to give rise to van der Waals repulsion. Thus these
cannot distinguish between enantiomers). It was estab- conformers are less stable (by 4 kJ/mole) than the anti
lished later that the bromine substituents are “staggered” conformer (Fig. 11B), in which the methyl groups are
rather than “eclipsed,” meaning that the torsion angle Br– remote from each other. [The relative instability of the
◦
◦
C–C–Br is 60 (Fig. 9D) or 180 (Fig. 9E) rather than 0 . gauche (Fig. 9D) relative to the anti confomer (Fig. 9E)
◦
The structures in Fig. 9D, E are said to differ in “confor- is even greater in BrCH 2 CH 2 Br because of additional
mation” (rotation about single bonds). dipole repulsion in Fig. 9D and its enantiomer.] The
Why are there differing isomers for 1,2-dibromoethane conformations of Fig. 11A, C in which the torsion angle
even though they cannot be isolated? The answer to this ω[C(1)–C(2)–C(3)–C(4)] is 60 ◦ are called “gauche”
FIGURE 10 Eclipsed and staggered conformations of ethane. Since the hydrogen atoms on the front carbon obscure
those in the rear in the eclipsed conformation, the torsion angle is offset by a few degrees in the Newman formula.
[Reprinted with permission from Eliel, E., Allinger, N. L., Morrison, G. A., and Angyal, S. J. (1981). “Conformational
Analysis,” American Chemical Society, Washington, DC. Copyright 1981 American Chemical Society.]