Page 5 - Academic Press Encyclopedia of Physical Science and Technology 3rd Organic Chemistry
P. 5
P1: GLQ Revised Pages
Encyclopedia of Physical Science and Technology EN001F-4 May 7, 2001 16:19
Acetylene 57
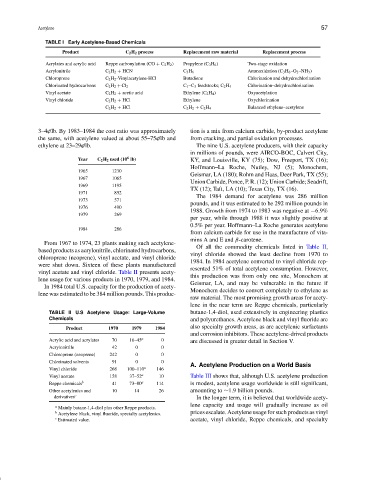
TABLE I Early Acetylene-Based Chemicals
Product C 2 H 2 process Replacement raw material Replacement process
Acrylates and acrylic acid Reppe carbonylation (CO + C 2 H 2 ) Propylene (C 3 H 6 ) Two-stage oxidation
Acrylonitrile C 2 H 2 + HCN C 3 H 6 Ammoxidation (C 3 H 6 –O 2 –NH 3 )
Chloroprene C 2 H 2 -Vinylacetylene-HCl Butadiene Chlorination and dehydrochlorination
Chlorinated hydrocarbons C 2 H 2 + Cl 2 C 1 –C 3 feedstocks; C 2 H 4 Chlorination–dehydrochlorination
Vinyl acetate C 2 H 2 + acetic acid Ethylene (C 2 H 4 ) Oxyacetylation
Vinyl chloride C 2 H 2 + HCl Ethylene Oxychlorination
C 2 H 2 + HCl C 2 H 2 + C 2 H 4 Balanced ethylene–acetylene
3–4c //lb. By 1983–1984 the cost ratio was approximately tion is a mix from calcium carbide, by-product acetylene
the same, with acetylene valued at about 55–75c //lb and from cracking, and partial oxidation processes.
ethylene at 23–29c //lb. The nine U.S. acetylene producers, with their capacity
in millions of pounds, were AIRCO-BOC, Calvert City,
6
Year C 2 H 2 used (10 lb) KY, and Louisville, KY (75); Dow, Freeport, TX (16);
Hoffmann–La Roche, Nutley, NJ (5); Monochem,
1965 1230
Geismar, LA (180); Rohm and Haas, Deer Park, TX (55);
1967 1065
Union Carbide, Ponce, P. R. (12); Union Carbide; Seadrift,
1969 1195
TX (12); Taft, LA (10); Texas City, TX (16).
1971 852
The 1984 demand for acetylene was 286 million
1973 571
pounds, and it was estimated to be 292 million pounds in
1976 490
1988. Growth from 1974 to 1983 was negative at −6.9%
1979 269
per year, while through 1988 it was slightly positive at
0.5% per year. Hoffmann–La Roche generates acetylene
1984 286
from calcium carbide for use in the manufacture of vita-
mins A and E and β-carotene.
From 1967 to 1974, 23 plants making such acetylene-
Of all the commodity chemicals listed in Table II,
based products as acrylonitrile, chlorinated hydrocarbons,
vinyl chloride showed the least decline from 1970 to
chloroprene (neoprene), vinyl acetate, and vinyl chloride
1984. In 1984 acetylene converted to vinyl chloride rep-
were shut down. Sixteen of these plants manufactured
resented 51% of total acetylene consumption. However,
vinyl acetate and vinyl chloride. Table II presents acety-
this production was from only one site, Monochem at
lene usage for various products in 1970, 1979, and 1984.
Geismar, LA, and may be vulnerable in the future if
In 1984 total U.S. capacity for the production of acety-
Monochem decides to convert completely to ethylene as
lene was estimated to be 384 million pounds. This produc-
raw material. The most promising growth areas for acety-
lene in the near term are Reppe chemicals, particularly
TABLE II U.S Acetylene Usage: Large-Volume butane-1,4-diol, used extensively in engineering plastics
Chemicals and polyurethanes. Acetylene black and vinyl fluoride are
also specialty growth areas, as are acetylenic surfactants
Product 1970 1979 1984
and corrosion inhibitors. These acetylene-drived products
Acrylic acid and acrylates 70 16–45 a 0 are discussed in greater detail in Section V.
Acrylonitrile 42 0 0
Chloroprene (neoprene) 242 0 0
Chlorinated solvents 91 0 0 A. Acetylene Production on a World Basis
Vinyl chloride 268 100–110 a 146
Vinyl acetate 158 37–52 a 10 Table III shows that, although U.S. acetylene production
Reppe chemicals b 41 73–80 a 114 is modest, acetylene usage worldwide is still significant,
Other acetylenics and 10 14 26 amounting to ∼1.9 billion pounds.
derivatives c In the longer term, it is believed that worldwide acety-
lene capacity and usage will gradually increase as oil
a
Mainly butane-1,4-diol plus other Reppe products.
b prices escalate. Acetylene usage for such products as vinyl
Acetylene black, vinyl fluoride, specialty acetylenics.
c
Estimated value. acetate, vinyl chloride, Reppe chemicals, and specialty