Page 57 - Academic Press Encyclopedia of Physical Science and Technology 3rd Organic Chemistry
P. 57
P1: ZCK 2nd Revised Pages
Encyclopedia of Physical Science and Technology EN002G-51 May 25, 2001 13:44
Bioconjugate Chemistry 95
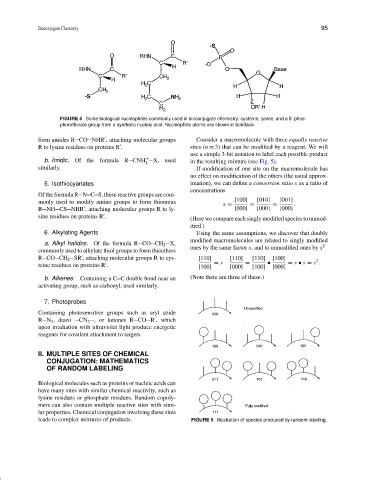
FIGURE 4 Some biological nucleophiles commonly used in bioconjugate chemistry: cysteine, lysine, and a 5 phos-
phorothioate group from a synthetic nucleic acid. Nucleophilic atoms are shown in boldface.
form amides R CO NHR , attaching molecular groups Consider a macromolecule with three equally reactive
R to lysine residues on proteins R . sites (n = 3) that can be modified by a reagent. We will
use a simple 3-bit notation to label each possible product
b. Imidic. Of the formula R CNH + X, used in the resulting mixture (see Fig. 5).
2
similarly. If modification of one site on the macromolecule has
no effect on modification of the others (the usual approx-
5. Isothiocyanates imation), we can define a conversion ratio s as a ratio of
concentrations
Of the formula R N C S, these reactive groups are com-
monly used to modify amino groups to form thioureas [100] [010] [001]
s = = = .
R NH CS NHR , attaching molecular groups R to ly- [000] [000] [000]
sine residues on proteins R .
(Here we compare each singly modified species to unmod-
ified.)
6. Alkylating Agents Using the same assumptions, we discover that doubly
modified macromolecules are related to singly modified
a. Alkyl halides. Of the formula R CO CH 2 X, 2
ones by the same factor s, and to unmodified ones by s
commonly used to alkylate thiol groups to form thioethers
R CO CH 2 SR , attaching molecular groups R to cys- [110] [110] [110] [100] 2
= s = • = s • s = s .
teine residues on proteins R . [100] [000] [100] [000]
b. Alkenes. Containing a C C double bond near an (Note there are three of these.)
activating group, such as carbonyl, used similarly.
7. Photoprobes
Containing photosensitive groups such as aryl azide
R N 3 , diazo CN 2 , or ketones R CO R , which
upon irradiation with ultraviolet light produce energetic
reagents for covalent attachment to targets.
II. MULTIPLE SITES OF CHEMICAL
CONJUGATION: MATHEMATICS
OF RANDOM LABELING
Biological molecules such as proteins or nucleic acids can
have many sites with similar chemical reactivity, such as
lysine residues or phosphate residues. Random copoly-
mers can also contain multiple reactive sites with simi-
lar properties. Chemical conjugation involving these sites
leads to complex mixtures of products. FIGURE 5 Illustration of species produced by random labeling.