Page 124 - Academic Press Encyclopedia of Physical Science and Technology 3rd Analytical Chemistry
P. 124
P1: GLQ/LSK P2: FQP Final Pages
Encyclopedia of Physical Science and Technology EN005E-212 June 15, 2001 20:32
Electron Spin Resonance 335
where m S and m I refer to the electron and nuclear spin
quantum numbers, respectively.
If we examine Fig. 3 we see that only two of the four
possible transitions are allowed, namely, those in which
the nuclear spin does not change its orientation. The en-
ergy difference between these two transitions is defined as
the hyperfine constant, usually symbolized by A in units of
megahertz (MHz) or gauss (G). Since
m I = 0, the effect
of the nuclear Zeeman term in the spin Hamiltonian will
always cancel out for first-order spectral transitions. Thus,
this term can be neglected in the Hamiltonian when one is
considering only first-order spectra. However, it should be
cautioned that if one considers spectra in which the per-
turbation theory approach must be carried out to second
order or if one considers spin relaxation, which will be
discussed later, the full spin Hamiltonian must be used.
Figure 4 shows the energy-level diagram based on
N
Eq. (8) for a hypothetical NH species where A and
+
H
H
N
A are assumed to be positive and A > A . Any sub-
script refers to the location of the nucleus in the molecule.
Figure 4 shows the energy levels of the spin system and FIGURE 5 Stick diagram for an ESR spectrum of hypothetical
+
the allowed transitions between sets of two energy levels. NH .
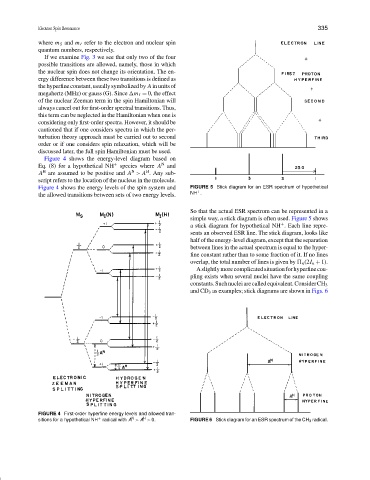
So that the actual ESR spectrum can be represented in a
simple way, a stick diagram is often used. Figure 5 shows
a stick diagram for hypothetical NH . Each line repre-
+
sents an observed ESR line. The stick diagram, looks like
half of the energy-level diagram, except that the separation
between lines in the actual spectrum is equal to the hyper-
fine constant rather than to some fraction of it. If no lines
overlap, the total number of lines is given by n (2I n + 1).
Aslightlymorecomplicatedsituationforhyperfinecou-
pling exists when several nuclei have the same coupling
constants.Suchnucleiarecalledequivalent.ConsiderCH 3
and CD 3 as examples; stick diagrams are shown in Figs. 6
FIGURE 4 First-order hyperfine energy levels and allowed tran-
H
N
sitions for a hypothetical NH radical with A > A > 0. FIGURE 6 Stick diagram for an ESR spectrum of the CH 3 radical.
+