Page 125 - Academic Press Encyclopedia of Physical Science and Technology 3rd Analytical Chemistry
P. 125
P1: GLQ/LSK P2: FQP Final Pages
Encyclopedia of Physical Science and Technology EN005E-212 June 15, 2001 20:32
336 Electron Spin Resonance
The assignment of an experimental coupling constant to
a given set of nuclei is sometimes not unique. Assignments
are usually made on the basis of theoretical calculations
or chemical substitutions. The spin density at a particular
proton is directly related to its coupling constant. Approx-
imate spin densities can be calculated by a simple H¨uckel
molecular orbital approach. They give a guide to the cou-
pling constant to be expected for a particular position. It
is more desirable if the experimental coupling constants
can be used to check the accuracy of the theoretical cal-
culation. Hence, assignments based on theoretical spin
densities should be used only when no direct information
can be obtained. Chemical substitutions can lead to an
unambiguous assignment. For example, deuterium can be
substituted for a given proton or set of equivalent protons.
Deuterium has a spin of 1 and a magnetic moment that
is 3.26 times smaller than that of H. Thus deuterium will
give a 6.5 times smaller splitting, which is sometimes not
even resolved. Methyl groups and Cl can also be substi-
tuted for certain protons to delineate the proper coupling
assignment.
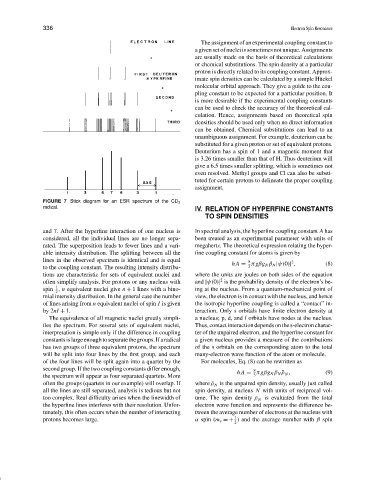
FIGURE 7 Stick diagram for an ESR spectrum of the CD 3
radical.
IV. RELATION OF HYPERFINE CONSTANTS
TO SPIN DENSITIES
and 7. After the hyperfine interaction of one nucleus is In spectral analysis, the hyperfine coupling constant A has
considered, all the individual lines are no longer sepa- been treated as an experimental parameter with units of
rated. The superposition leads to fewer lines and a vari- megahertz. The theoretical expression relating the hyper-
able intensity distribution. The splitting between all the fine coupling constant for atoms is given by
lines in the observed spectrum is identical and is equal 8 2
hA = πgβg N β N |ψ(0)| , (8)
to the coupling constant. The resulting intensity distribu- 3
tions are characteristic for sets of equivalent nuclei and where the units are joules on both sides of the equation
2
often simplify analysis. For protons or any nucleus with and |ψ(0)| is the probability density of the electron’s be-
1
spin , n equivalent nuclei give n + 1 lines with a bino- ing at the nucleus. From a quantum-mechanical point of
2
mial intensity distribution. In the general case the number view, the electron is in contact with the nucleus, and hence
of lines arising from n equivalent nuclei of spin I is given the isotropic hyperfine coupling is called a “contact” in-
by 2nI + 1. teraction. Only s orbitals have finite electron density at
The equivalence of all magnetic nuclei greatly simpli- a nucleus; p, d, and f orbitals have nodes at the nucleus.
fies the spectrum. For several sets of equivalent nuclei, Thus,contactinteractiondependsonthes-electroncharac-
interpretation is simple only if the difference in coupling ter of the unpaired electron, and the hyperfine constant for
constantsislargeenoughtoseparatethegroups.Ifaradical a given nucleus provides a measure of the contributions
has two groups of three equivalent protons, the spectrum of the s orbitals on the corresponding atom to the total
will be split into four lines by the first group, and each many-electron wave function of the atom or molecule.
of the four lines will be split again into a quartet by the For molecules, Eq. (8) can be rewritten as
second group. If the two coupling constants differ enough, 8
hA = πgβg N β N ¯ρ , (9)
the spectrum will appear as four separated quartets. More 3 N
often the groups (quartets in our example) will overlap. If where ¯ρ is the unpaired spin density, usually just called
N
all the lines are still separated, analysis is tedious but not spin density, at nucleus N with units of reciprocal vol-
too complex. Real difficulty arises when the linewidth of ume. The spin density ¯ρ is evaluated from the total
N
the hyperfine lines interferes with their resolution. Unfor- electron wave function and represents the difference be-
tunately, this often occurs when the number of interacting tween the average number of electrons at the nucleus with
1
protons becomes large. α spin (m s =+ ) and the average number with β spin
2