Page 304 - Academic Press Encyclopedia of Physical Science and Technology 3rd Analytical Chemistry
P. 304
P1: GNH Final Pages
Encyclopedia of Physical Science and Technology EN009N-447 July 19, 2001 23:3
Microwave Molecular Spectroscopy 821
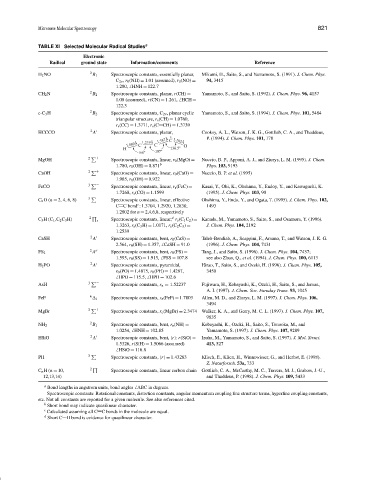
TABLE XI Selected Molecular Radical Studies a
Electronic
Radical ground state Information/comments Reference
H 2 NO 2 B 1 Spectroscopic constants, essentially planar, Mikami, H., Saito, S., and Yamamoto, S. (1991). J. Chem. Phys.
C 2v , r 0 (NH) = 1.01 (assumed), r 0 (NO) = 94, 3415
1.280, HNH = 122.7
CH 2 N 2 B 2 Spectroscopic constants, planar, r(CH) = Yamamoto, S., and Saito, S. (1992). J. Chem. Phys. 96, 4157
1.08 (assumed), r(CN) = 1.261, HCH =
122.3
c-C 3 H 2 B 2 Spectroscopic constants, C 2v , planar cyclic Yamamoto, S., and Saito, S. (1994). J. Chem. Phys. 101, 5484
triangular structure, r s (CH) = 1.0760,
r s (CC) = 1.3771, r s (C CH) = 1.3739
HCCCO 2 A Spectroscopic constants, planar, Cooksy, A. L., Watson, J. K. G., Gottlieb, C. A., and Thaddeus,
1.387Å C 1.192Å P. (1994). J. Chem. Phys. 101, 178
1.060Å C 1.219Å C O
H 136.5
168 197
MgOH 2 + Spectroscopic constants, linear, r 0 (MgO) = Nuccio, B. P., Apponi, A. J., and Ziurys, L. M. (1995). J. Chem.
1.780, r 0 (OH) = 0.871 b Phys. 103, 9193
CaOH 2 + Spectroscopic constants, linear, r 0 (CaO) = Nuccio, B. P. et al. (1995)
1.985, r 0 (OH) = 0.922
FeCO 3 − Spectroscopic constants, linear, r s (FeC) = Kasai, Y., Obi, K., Ohshima, Y., Endoy, Y., and Kawaguchi, K.
1.7268, r s (CO) = 1.1599 (1995). J. Chem. Phys. 103, 90
C n O(n = 2, 4, 6, 8) 3 − Spectroscopic constants, linear, effective Ohshima, Y., Endo, Y., and Ogata, T. (1995). J. Chem. Phys. 102,
c
C C bond :1.3704, 1.2920, 1.2830, 1493
1.2802 for n = 2,4,6,8, respectively
d
C 3 H(C 1 ,C 2 C 3 H) 2 r Spectroscopic constants, linear, r s (C 1 C 2 ) = Kanada, M., Yamamoto, S., Saito, S., and Osamura, Y. (1996).
1.3263, r s (C 3 H) = 1.0171, r s (C 2 C 3 ) = J. Chem. Phys. 104, 2192
1.2539
CaSH 2 A Spectroscopic constants, bent, r 0 (CaS) = Taleb-Bendiab, A., Scappini, F., Amano, T., and Watson, J. K. G.
2.564, r 0 (SH) = 1.357, CaSH = 91.0 (1996). J. Chem. Phys. 104, 7431
2
FS 2 A Spectroscopic constants, bent, r 0 (FS) = Tang, J., and Saito, S. (1996). J. Chem. Phys. 104, 7437;
1.595, r 0 (SS) = 1.915, FSS = 107.8 see also Zhuo, Q., et al. (1994). J. Chem. Phys. 100, 6113
H 2 PO 2 A Spectroscopic constants, pyramidal, Hirao, T., Saito, S., and Ozeki, H. (1996). J. Chem. Phys. 105,
r 0 (PO) = 1.4875, r 0 (PH) = 1.4287, 3450
HPO = 115.5, HPH = 102.6
AsH 3 − Spectroscopic constants, r e = 1.52237 Fujiwara, H., Kobayashi, K., Ozeki, H., Saito, S., and Jaman,
A. I. (1997). J. Chem. Soc. Faraday Trans. 93, 1045
FeF 6 i Spectroscopic constants, r e (FeF) = 1.7803 Allen, M. D., and Ziurys, L. M. (1997). J. Chem. Phys. 106,
3494
MgBr 2 + Spectroscopic constants, r e (MgBr) = 2.3474 Walker, K. A., and Gerry, M. C. L. (1997). J. Chem. Phys. 107,
9835
2
NH 2 B 1 Spectroscopic constants, bent, r e (NH) = Kobayashi, K., Ozeki, H., Saito, S., Tonooka, M., and
1.0254, HNH = 102.85 Yamamoto, S. (1997). J. Chem. Phys. 107, 9289
HSiO 2 A Spectroscopic constants, bent, r :r(SiO) = Izuha, M., Yamamoto, S., and Saito, S. (1997). J. Mol. Struct.
1.5326, r(SiH) = 1.5066 (assumed) 413, 527
HSiO = 116.8
PH 3 − Spectroscopic constants, r = 1.43283 Klisch, E., Klien, H., Winnewisser, G., and Herbst, E. (1998).
Z. Naturforsch. 53a, 733
C n H(n = 10, 2 Spectroscopic constants, linear carbon chain Gottlieb, C. A., McCarthy, M. C., Travers, M. J., Grabow, J.-U.,
12,13,14) and Thaddeus, P. (1998). J. Chem. Phys. 109, 5433
a Bond lengths in angstrom units, bond angles ABC in degrees.
Spectroscopic constants: Rotational constants, distortion constants, angular momentum coupling fine structure terms, hyperfine coupling constants,
etc. Not all constants are reported for a given molecule. See also references cited.
b Short bond may indicate quasilinear character.
c Calculated assuming all C C bonds in the molecule are equal.
d Short C H bond is evidence for quasilinear character.