Page 330 - Academic Press Encyclopedia of Physical Science and Technology 3rd Analytical Chemistry
P. 330
P1: GNH Final Pages
Encyclopedia of Physical Science and Technology EN009N-447 July 19, 2001 23:3
Microwave Molecular Spectroscopy 847
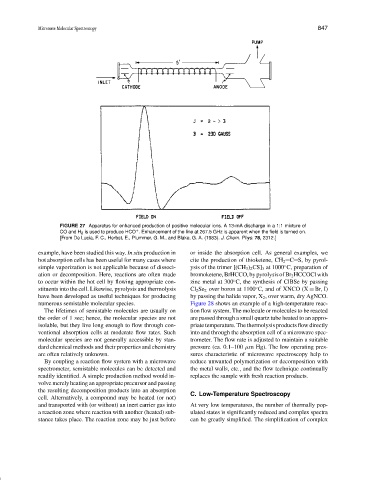
FIGURE 27 Apparatus for enhanced production of positive molecular ions. A 13-mA discharge in a 1:1 mixture of
CO and H 2 is used to produce HCO . Enhancement of the line at 267.5 GHz is apparent when the field is turned on.
+
[From De Lucia, F. C., Herbst, E., Plummer, G. M., and Blake, G. A. (1983). J. Chem. Phys. 78, 2312.]
example, have been studied this way. In situ production in or inside the absorption cell. As general examples, we
hot absorption cells has been useful for many cases where cite the production of thioketene, CH 2 C S, by pyrol-
◦
simple vaporization is not applicable because of dissoci- ysis of the trimer [(CH 3 ) 2 CS] 3 at 1000 C, preparation of
ation or decomposition. Here, reactions are often made bromoketene, BrHCCO, by pyrolysis of Br 2 HCCOCl with
to occur within the hot cell by flowing appropriate con- zinc metal at 300 C, the synthesis of ClBSe by passing
◦
◦
stituents into the cell. Likewise, pyrolysis and thermolysis Cl 2 Se 2 over boron at 1100 C, and of XNCO (X = Br, I)
have been developed as useful techniques for producing by passing the halide vapor, X 2 , over warm, dry AgNCO.
numerous semistable molecular species. Figure 28 shows an example of a high-temperature reac-
The lifetimes of semistable molecules are usually on tion flow system. The molecule or molecules to be reacted
the order of 1 sec; hence, the molecular species are not are passed through a small quartz tube heated to an appro-
isolable, but they live long enough to flow through con- priate temperature. The thermolysis products flow directly
ventional absorption cells at moderate flow rates. Such into and through the absorption cell of a microwave spec-
molecular species are not generally accessible by stan- trometer. The flow rate is adjusted to maintain a suitable
dard chemical methods and their properties and chemistry pressure (ca. 0.1–100 µm Hg). The low operating pres-
are often relatively unknown. sures characteristic of microwave spectroscopy help to
By coupling a reaction flow system with a microwave reduce unwanted polymerization or decomposition with
spectrometer, semistable molecules can be detected and the metal walls, etc., and the flow technique continually
readily identified. A simple production method would in- replaces the sample with fresh reaction products.
volve merely heating an appropriate precursor and passing
the resulting decomposition products into an absorption
C. Low-Temperature Spectroscopy
cell. Alternatively, a compound may be heated (or not)
and transported with (or without) an inert carrier gas into At very low temperatures, the number of thermally pop-
a reaction zone where reaction with another (heated) sub- ulated states is significantly reduced and complex spectra
stance takes place. The reaction zone may be just before can be greatly simplified. The simplification of complex