Page 395 - Academic Press Encyclopedia of Physical Science and Technology 3rd Analytical Chemistry
P. 395
P1: GTQ/GLE P2: GPJ Final Pages
Encyclopedia of Physical Science and Technology EN012C-562 July 26, 2001 15:30
2 Photoacoustic Spectroscopy
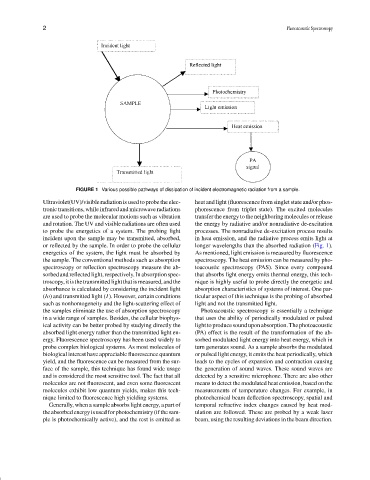
FIGURE 1 Various possible pathways of dissipation of incident electromagnetic radiation from a sample.
Ultraviolet(UV)/visible radiation is used to probe the elec- heat and light (fluorescence from singlet state and/or phos-
tronic transitions, while infrared and microwave radiations phorescence from triplet state). The excited molecules
are used to probe the molecular motions such as vibration transfer the energy to the neighboring molecules or release
and rotation. The UV and visible radiations are often used the energy by radiative and/or nonradiative de-excitation
to probe the energetics of a system. The probing light processes. The nonradiative de-excitation process results
incident upon the sample may be transmitted, absorbed, in heat emission, and the radiative process emits light at
or reflected by the sample. In order to probe the cellular longer wavelengths than the absorbed radiation (Fig. 1).
energetics of the system, the light must be absorbed by As mentioned, light emission is measured by fluorescence
the sample. The conventional methods such as absorption spectroscopy. The heat emission can be measured by pho-
spectroscopy or reflection spectroscopy measure the ab- toacoustic spectroscopy (PAS). Since every compound
sorbedandreflectedlight,respectively.Inabsorptionspec- that absorbs light energy emits thermal energy, this tech-
troscopy,itisthetransmittedlightthatismeasured,andthe nique is highly useful to probe directly the energetic and
absorbance is calculated by considering the incident light absorption characteristics of systems of interest. One par-
(Io) and transmitted light (I). However, certain conditions ticular aspect of this technique is the probing of absorbed
such as nonhomogeneity and the light-scattering effect of light and not the transmitted light.
the samples eliminate the use of absorption spectroscopy Photoacoustic spectroscopy is essentially a technique
in a wide range of samples. Besides, the cellular biophys- that uses the ability of periodically modulated or pulsed
ical activity can be better probed by studying directly the lighttoproducesounduponabsorption.Thephotoacoustic
absorbed light energy rather than the transmitted light en- (PA) effect is the result of the transformation of the ab-
ergy. Fluorescence spectroscopy has been used widely to sorbed modulated light energy into heat energy, which in
probe complex biological systems. As most molecules of turn generates sound. As a sample absorbs the modulated
biological interest have appreciable fluorescence quantum or pulsed light energy, it emits the heat periodically, which
yield, and the fluorescence can be measured from the sur- leads to the cycles of expansion and contraction causing
face of the sample, this technique has found wide usage the generation of sound waves. These sound waves are
and is considered the most sensitive tool. The fact that all detected by a sensitive microphone. There are also other
molecules are not fluorescent, and even some fluorescent means to detect the modulated heat emission, based on the
molecules exhibit low quantum yields, makes this tech- measurements of temperature changes. For example, in
nique limited to fluorescence high yielding systems. photochemical beam deflection spectroscopy, spatial and
Generally, when a sample absorbs light energy, a part of temporal refractive index changes caused by heat mod-
theabsorbedenergyisusedforphotochemistry(ifthesam- ulation are followed. These are probed by a weak laser
ple is photochemically active), and the rest is emitted as beam, using the resulting deviations in the beam direction.