Page 7 - Academic Press Encyclopedia of Physical Science and Technology 3rd Analytical Chemistry
P. 7
P1: GJB Revised Pages
Encyclopedia of Physical Science and Technology En001f25 May 7, 2001 13:58
546 Analytical Chemistry
methods (see later). Accordingly, it is still frequently used ion, bases are standardized against potassium hydrogen
as a “standardizing” technique for instrumental methods. phthalate (KHC 8 H 4 O 4 ). The end point in a strong acid–
Gravimetry, however, can be rather time consuming, es- strong base neutralization titration is usually found from
pecially if a large number of samples are involved. the in situ behavior of an added indicator, which is gener-
ally a weak organic acid or base that undergoes chemical
changes exhibiting different colors. For example, we can
C. Titrimetric (Volumetric) Analysis
write for the acid-type indicator HIn
In titrimetric analysis, which is often termed volumetric
−
H 2 O + HIn = H 3 O + In ,
+
analysis, we obtain the volume of a standard reagent re-
quired to consume an analyte completely. On a practical (color 1 in (color 2 in
basis a standard solution of reagent, the concentration of acid solution) basic solution)
which is known accurately, is added by a buret until it is
Thus, it is very important to know the range of hydrogen
decided that the analyte is just used up. This condition is
ion concentration (i.e., pH) in which a change from color
usually called the equivalence point. Since it is difficult
1 to color 2 can be observed. Generally, this occurs within
to observe this point experimentally, it is usually approx-
approximately ±1 pH unit of the pK a of the indicator.
imated by the distinction of an end point, which is asso-
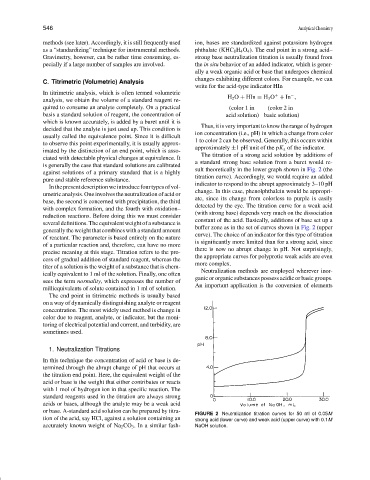
The titration of a strong acid solution by additions of
ciated with detectable physical changes at equivalence. It
a standard strong base solution from a buret would re-
is generally the case that standard solutions are calibrated
sult theoretically in the lower graph shown in Fig. 2 (the
against solutions of a primary standard that is a highly
titration curve). Accordingly, we would require an added
pure and stable reference substance.
indicator to respond to the abrupt approximately 3–10 pH
Inthepresentdescriptionweintroducefourtypesofvol-
change. In this case, phenolphthalein would be appropri-
umetric analysis. One involves the neutralization of acid or
ate, since its change from colorless to purple is easily
base, the second is concerned with precipitation, the third
detected by the eye. The titration curve for a weak acid
with complex formation, and the fourth with oxidation–
(with strong base) depends very much on the dissociation
reduction reactions. Before doing this we must consider
constant of the acid. Basically, additions of base set up a
several definitions. The equivalent weight of a substance is
buffer zone as in the set of curves shown in Fig. 2 (upper
generallytheweightthatcombineswithastandardamount
curve). The choice of an indicator for this type of titration
of reactant. The parameter is based entirely on the nature
is significantly more limited than for a strong acid, since
of a particular reaction and, therefore, can have no more
there is now no abrupt change in pH. Not surprisingly,
precise meaning at this stage. Titration refers to the pro-
the appropriate curves for polyprotic weak acids are even
cess of gradual addition of standard reagent, whereas the
more complex.
titer of a solution is the weight of a substance that is chem-
Neutralization methods are employed wherever inor-
ically equivalent to 1 ml of the solution. Finally, one often
ganic or organic substances possess acidic or basic groups.
sees the term normality, which expresses the number of
An important application is the conversion of elements
milliequivalents of solute contained in 1 ml of solution.
The end point in titrimetric methods is usually based
on a way of dynamically distinguishing analyte or reagent
concentration. The most widely used method is change in
color due to reagent, analyte, or indicator, but the moni-
toring of electrical potential and current, and turbidity, are
sometimes used.
1. Neutralization Titrations
In this technique the concentration of acid or base is de-
termined through the abrupt change of pH that occurs at
the titration end point. Here, the equivalent weight of the
acid or base is the weight that either contributes or reacts
with 1 mol of hydrogen ion in that specific reaction. The
standard reagents used in the titration are always strong
acids or bases, although the analyte may be a weak acid
or base. A-standard acid solution can be prepared by titra-
FIGURE 2 Neutralization titration curves for 50 ml of 0.05M
tion of the acid, say HCl, against a solution containing an strong acid (lower curve) and weak acid (upper curve) with 0.1M
accurately known weight of Na 2 CO 3 . In a similar fash- NaOH solution.