Page 95 - Academic Press Encyclopedia of Physical Science and Technology 3rd Analytical Chemistry
P. 95
P1: GRB Final Pages
Encyclopedia of Physical Science and Technology EN005M-206 June 15, 2001 20:25
Electrochemistry 173
II
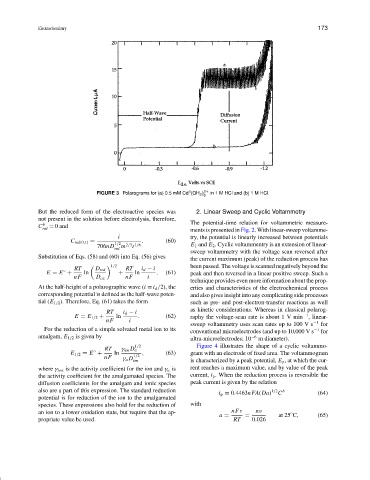
FIGURE 3 Polarograms for (a) 0.5 mM Cd (OH 2 ) 2+ in 1 M HCl and (b) 1 M HCl.
6
But the reduced form of the electroactive species was 2. Linear Sweep and Cyclic Voltammetry
not present in the solution before electrolysis, therefore, The potential-time relation for voltammetric measure-
C b = 0 and
red ments is presented in Fig. 2. With linear-sweep voltamme-
i try, the potential is linearly increased between potentials
C red(0,t) = 1/2 . (60)
t
706nD m 2/3 1/6 E 1 and E 2 . Cyclic voltammentry is an extension of linear-
red
sweep voltammetry with the voltage scan reversed after
Substitution of Eqs. (58) and (60) into Eq. (56) gives the current maximum (peak) of the reduction process has
1/2 been passed. The voltage is scanned negatively beyond the
RT D red RT i d − i
◦
E = E + ln + ln . (61) peak and then reversed in a linear positive sweep. Such a
nF D ox nF i
technique provides even more information about the prop-
At the half-height of a polarographic wave (i = i d /2), the erties and characteristics of the electrochemical process
corresponding potential is defined as the half-wave poten- and also gives insight into any complicating side processes
tial (E 1/2 ). Therefore, Eq. (61) takes the form such as pre- and post-electron-transfer reactions as well
as kinetic considerations. Whereas in classical polarog-
RT i d − i
−1
E = E 1/2 + ln . (62) raphy the voltage-scan rate is about 1 V min , linear-
nF i −1
sweep voltammetry uses scan rates up to 100 V s for
For the reduction of a simple solvated metal ion to its −1
conventional microelectrodes (and up to 10,000 V s for
amalgam, E 1/2 is given by −6
ultra-microelectrodes; 10 m diameter).
1/2 Figure 4 illustrates the shape of a cyclic voltammo-
RT γ ion D a
◦
E 1/2 = E + ln 1/2 , (63) gram with an electrode of fixed area. The voltammogram
nF γ a D
ion is characterized by a peak potential, E p , at which the cur-
where γ ion is the activity coefficient for the ion and γ a is rent reaches a maximum value, and by value of the peak
the activity coefficient for the amalgamated species. The current, i p . When the reduction process is reversible the
diffusion coefficients for the amalgam and ionic species peak current is given by the relation
also are a part of this expression. The standard reduction 1/2 b
i p = 0.4463nFA(Da) C (64)
potential is for reduction of the ion to the amalgamated
species. These expressions also hold for the reduction of with
an ion to a lower oxidation state, but require that the ap- nFν nν
◦
a = = at 25 C, (65)
propriate value be used. RT 0.026