Page 100 - Academic Press Encyclopedia of Physical Science and Technology 3rd Analytical Chemistry
P. 100
P1: GRB Final Pages
Encyclopedia of Physical Science and Technology EN005M-206 June 15, 2001 20:25
178 Electrochemistry
TABLE II Oxidation Potentials for Ligands (L) and Their ML 3
Complexes with Zn(II), Mn(II), Fe(II), and Co(II) in MeCN (0.1 M
Tetraethylammonium Perchlorate)
E b ,VvsNHE c
Ligand 1/2
+
II
II
− a
II
II
−
(L or L ) L/L · (L /L·) Zn L 3 Mn L 3 Fe L 3 Co L 3
H 2 O +2.8 +2.8 +2.8 +1.84 +2.8
bpy +2.32 >+2.5 +1.55 +1.30 +0.58
PA − +1.50 +1.54 +0.60 +0.20 +0.04
acac − +0.55 +0.58 +0.18 −0.42 −0.35
8-Q − +0.21 +0.22 −0.06 −0.41 −0.57
a −
Key: bpy, 2,2 -bipyridine; PA , picolinate (2-carboxylate pyridine);
−
−
acac , acetylacetonate; 8-Q , 8-quinolinate.
b II
E 1/2 taken as (E p,a + E p,c )/2 for reversible couples of Mn L 3 and
II
II
Fe L 3 complexes; as E p,a/2 + 0.03 V for L (or L ) and Zn L 3 ; and
−
II
as E p,c/2 − 0.03 V for the Co L 3 complex that exhibits separated redox
couples.
c SCE vs NHE; +0.242 V.
Table II summarizes the oxidation potentials for lig-
II
ands (L) and their M L 3 complexes with zinc(II), man-
ganese(II), iron(II), and cobalt(II). The difference in the
potentials for the free and complexed ligands is a measure
of the metal(III)-ligand covalent-bond-formation energies
(− G BF ); these are summarized in Table III. All of the
data are consistent with ligand-centered redox processes.
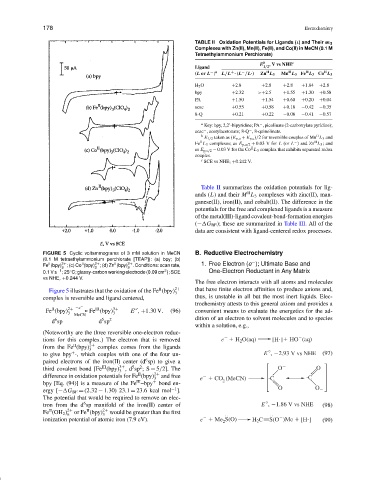
FIGURE 5 Cyclic voltammograms of 3 mM solution in MeCN B. Reductive Electrochemistry
(0.1 M tetraethylammonium perchlorate [TEAP]): (a) bpy; (b)
−
II
II
II
Fe (bpy) 2+ ; (c) Co (bpy) 2+ ; (d) Zn (bpy) 2+ . Conditions: scan rate, 1. Free Electron (e ); Ultimate Base and
3 3 3
2
◦
0.1 V s −1 ;25 C; glassy-carbon working electrode (0.09 cm ); SCE One-Electron Reductant in Any Matrix
vs NHE, +0.244 V.
The free electron interacts with all atoms and molecules
II
Figure 5 illustrates that the oxidation of the Fe (bpy) 2+ that have finite electron affinities to produce anions and,
3
complex is reversible and ligand centered, thus, is unstable in all but the most inert liquids. Elec-
trochemistry attests to this general axiom and provides a
−
II
III
◦
Fe (bpy) 2+ −e Fe (bpy) 3+ E , +1.30 V. (96) convenient means to evaluate the energetics for the ad-
3 3
MeCN
5
6
d sp d sp 2 dition of an electron to solvent molecules and to species
within a solution, e.g.,
(Noteworthy are the three reversible one-electron reduc-
tions for this complex.) The electron that is removed e H 2 O(aq) [H ] HO (aq)
II
from the Fe (bpy) 2+ complex comes from the ligands
3
°
+
to give bpy ·, which couples with one of the four un- E , 2.93 V vs NHE (97)
6
paired electrons of the iron(II) center (d sp) to give a
III
5
2
3+
third covalent bond [Fe (bpy) ,d sp ;S = 5/2]. The O O
3
II
difference in oxidation potentials for Fe (bpy) 2+ and free
3 e CO (MeCN) C C
III
bpy [Eq. (94)] is a measure of the Fe –bpy bond en- 2
+
−1
ergy [− G BF = (2.32 − 1.30) 23.1 = 23.6 kcal mol ]. O O
The potential that would be required to remove an elec-
°
6
tron from the d sp manifold of the iron(II) center of E , 1.86 V vs NHE (98)
II
II
Fe (OH 2 ) 2+ or Fe (bpy) 2+ would be greater than the first
6 3
ionization potential of atomic iron (7.9 eV). e Me S(O) H 2 C S(O )Me [H ] (99)
2