Page 70 - Academic Press Encyclopedia of Physical Science and Technology 3rd BioChemistry
P. 70
P1: GTQ/GUU P2: GLM Final Pages
Encyclopedia of Physical Science and Technology EN008K-353 June 29, 2001 12:41
Ion Transport Across Biological Membranes 103
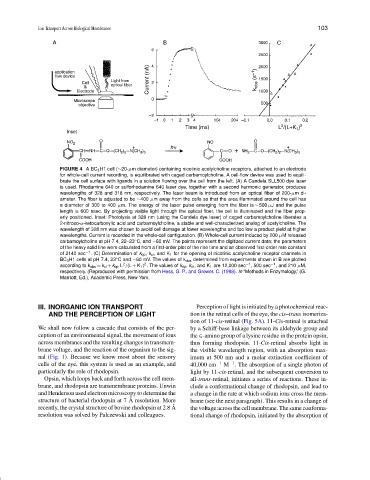
FIGURE 4 ABC 3 H1 cell (∼20-µm diameter) containing nicotinic acetylcholine receptors, attached to an electrode
for whole-cell current recording, is equilibrated with caged carbamoylcholine. A cell-flow device was used to equili-
brate the cell surface with ligands in a solution flowing over the cell from the left. (A) A Candela SLL500 dye laser
is used. Rhodamine 640 or sulforhodamine 640 laser dye, together with a second harmonic generator, produces
wavelengths of 328 and 318 nm, respectively. The laser beam is introduced from an optical fiber of 200-µm di-
ameter. The fiber is adjusted to be ∼400 µm away from the cells so that the area illuminated around the cell has
a diameter of 300 to 400 µm. The energy of the laser pulse emerging from the fiber is ∼ 500 µJ and the pulse
length is 600 nsec. By projecting visible light through the optical fiber, the cell is illuminated and the fiber prop-
erly positioned. Inset: Photolysis at 328 nm (using the Candela dye laser) of caged carbamoylcholine liberates a
2-nitroso-α-ketocarboxylic acid and carbamoylcholine, a stable and well-characterized analog of acetylcholine. The
wavelength of 328 nm was chosen to avoid cell damage at lower wavelengths and too low a product yield at higher
wavelengths. Current is recorded in the whole-cell configuration. (B) Whole-cell current induced by 200 µM released
carbamoylcholine at pH 7.4, 22–23 C, and −60 mV. The points represent the digitized current data; the parameters
◦
of the heavy solid line were calculated from a first-order plot of the rise time and an observed first-order rate constant
of 2140 sec −1 . (C) Determination of k op , k cl , and K 1 for the opening of nicotinic acetylcholine receptor channels in
BC 3 H1 cells at pH 7.4, 23 C and −60 mV. The values of k obs determined from experiments shown in B are plotted
◦
2
2
according to k obs = k cl + k op L /(L + K 1 ) . The values of k op , k cl , and K 1 are 12,000 sec −1 , 500 sec −1 , and 210 µM,
respectively. (Reproduced with permission from Hess, G. P., and Grewer, C. (1998). In “Methods in Enzymology,” (G.
Marriott, Ed.), Academic Press, New York.
III. INORGANIC ION TRANSPORT Perception of light is initiated by a photochemical reac-
AND THE PERCEPTION OF LIGHT tion in the retinal cells of the eye, the cis–trans isomeriza-
tion of 11-cis-retinal (Fig. 5A). 11-Cis-retinal is attached
We shall now follow a cascade that consists of the per- by a Schiff-base linkage between its aldehyde group and
ception of an environmental signal, the movement of ions the ∈-amino group of a lysine residue in the protein opsin,
across membranes and the resulting changes in transmem- thus forming rhodopsin. 11-Cis-retinal absorbs light in
brane voltage, and the reaction of the organism to the sig- the visible wavelength region, with an absorption max-
nal (Fig. 1). Because we know most about the sensory imum at 500 nm and a molar extinction coefficient of
−1
cells of the eye, this system is used as an example, and 40,000 cm −1 M . The absorption of a single photon of
particularly the role of rhodopsin. light by 11-cis-retinal, and the subsequent conversion to
Opsin, which loops back and forth across the cell mem- all-trans-retinal, initiates a series of reactions. These in-
brane, and rhodopsin are transmembrane proteins. Unwin clude a conformational change of rhodopsin, and lead to
and Henderson used electron microscopy to determine the a change in the rate at which sodium ions cross the mem-
˚
structure of bacterial rhodopsin at 7 A resolution. More brane (see the next paragraph). This results in a change of
˚
recently, the crystal structure of bovine rhodopsin at 2.8 A the voltage across the cell membrane. The same conforma-
resolution was solved by Palczewski and colleagues. tional change of rhodopsin, initiated by the absorption of