Page 24 - Academic Press Encyclopedia of Physical Science and Technology 3rd Polymer
P. 24
P1: FPP 2nd Revised Pages
Encyclopedia of Physical Science and Technology EN002C-64 May 19, 2001 20:39
228 Biopolymers
are incompletely digested (or not at all) in the small intes-
tine but may be fermented by resident bacteria in the large
intestine. It is now believed that such polysaccharides are
beneficial to human health in that they seem to contribute
to the lowering of blood cholesterol levels and thus help
to reduce heart disease. They modulate the absorption of
glucose into the bloodstream, and possibly even lower the
incidence of bowel cancer.
Some of the most complex polysaccharides known are
found in gums that are exuded as a viscous fluid from some
plants, often at the site of an injury. The fluid then hard-
ens to a clear nodule consisting mainly of polysaccharide,
which may have a protective function to prevent further
damage to the plant. The major polysaccharide of gum ara-
bic, for example, has a backbone of galactose residues, to
which are attached branches containing arabinose, rham-
nose, galactose, and glucuronic acid residues. Gums give
highly viscous solutions or gels with water and so are used
as thickening agents and binding agents in the food and
pharmaceutical industries.
4. Marine Polysaccharides
Whereas in land plants polysaccharide structures often
appear irregular, with an apparently random distribution
of branches or monomers along a main chain, the
structures of some seaweed polysaccharides are based
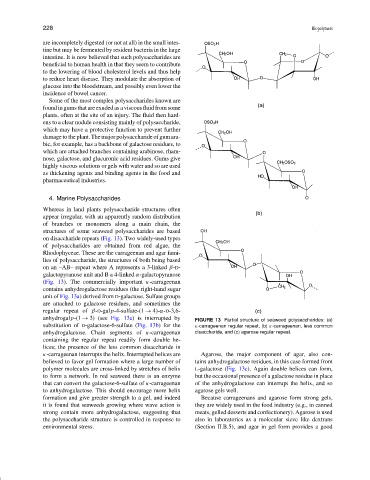
on disaccharide repeats (Fig. 13). Two widely-used types
of polysaccharides are obtained from red algae, the
Rhodophyceae. These are the carrageenan and agar fami-
lies of polysaccharide, the structures of both being based
on an –AB– repeat where A represents a 3-linked β-D-
galactopyranose unit and B a 4-linked α-galactopyranose
(Fig. 13). The commercially important κ-carrageenan
contains anhydrogalactose residues (the right-hand sugar
unit of Fig. 13a) derived from D-galactose. Sulfate groups
are attached to galactose residues, and sometimes the
regular repeat of β-D-galp-4-sulfate-(1 → 4)-α-D-3,6-
anhydrogalp-(1 → 3) (see Fig. 13a) is interrupted by FIGURE 13 Partial structure of seaweed polysaccharides: (a)
substitution of D-galactose-6-sulfate (Fig. 13b) for the κ-carrageenan regular repeat, (b) κ-carrageenan, less common
anhydrogalactose. Chain segments of κ-carrageenan disaccharide, and (c) agarose regular repeat.
containing the regular repeat readily form double he-
lices; the presence of the less common disaccharide in
κ-carrageenan interrupts the helix. Interrupted helices are Agarose, the major component of agar, also con-
believed to favor gel formation where a large number of tains anhydrogalactose residues, in this case formed from
polymer molecules are cross-linked by stretches of helix L-galactose (Fig. 13c). Again double helices can form,
to form a network. In red seaweed there is an enzyme but the occasional presence of a galactose residue in place
that can convert the galactose-6-sulfate of κ-carrageenan of the anhydrogalactose can interrupt the helix, and so
to anhydrogalactose. This should encourage more helix agarose gels well.
formation and give greater strength to a gel, and indeed Because carrageenans and agarose form strong gels,
it is found that seaweeds growing where wave action is they are widely used in the food industry (e.g., in canned
strong contain more anhydrogalactose, suggesting that meats, gelled desserts and confectionery). Agarose is used
the polysaccharide structure is controlled in response to also in laboratories as a molecular sieve like dextrans
environmental stress. (Section II.B.5), and agar in gel form provides a good