Page 29 - Academic Press Encyclopedia of Physical Science and Technology 3rd Polymer
P. 29
P1: FPP 2nd Revised Pages
Encyclopedia of Physical Science and Technology EN002C-64 May 19, 2001 20:39
Biopolymers 233
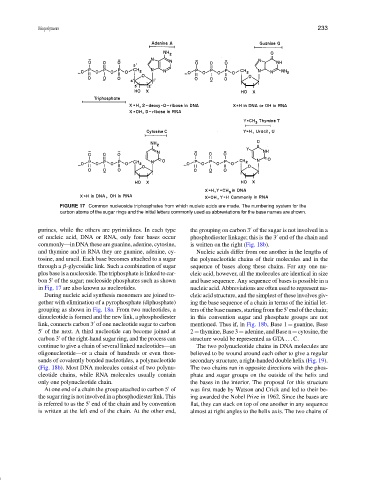
FIGURE 17 Common nucleoside triphosphates from which nucleic acids are made. The numbering system for the
carbon atoms of the sugar rings and the initial letters commonly used as abbreviations for the base names are shown.
purines, while the others are pyrimidines. In each type the grouping on carbon 3 of the sugar is not involved in a
of nucleic acid, DNA or RNA, only four bases occur phosphodiester linkage; this is the 3 end of the chain and
commonly—in DNA these are guanine, adenine, cytosine, is written on the right (Fig. 18b).
and thymine and in RNA they are guanine, adenine, cy- Nucleic acids differ from one another in the lengths of
tosine, and uracil. Each base becomes attached to a sugar the polynucleotide chains of their molecules and in the
through a β-glycosidic link. Such a combination of sugar sequence of bases along these chains. For any one nu-
plus base is a nucleoside. The triphosphate is linked to car- cleic acid, however, all the molecules are identical in size
bon 5 of the sugar; nucleoside phosphates such as shown and base sequence. Any sequence of bases is possible in a
in Fig. 17 are also known as nucleotides. nucleic acid. Abbreviations are often used to represent nu-
During nucleic acid synthesis monomers are joined to- cleic acid structure, and the simplest of these involves giv-
gether with elimination of a pyrophosphate (diphosphate) ing the base sequence of a chain in terms of the initial let-
grouping as shown in Fig. 18a. From two nucleotides, a ters of the base names, starting from the 5 end of the chain;
dinucleotide is formed and the new link, a phosphodiester in this convention sugar and phosphate groups are not
link, connects carbon 3 of one nucleotide sugar to carbon mentioned. Thus if, in Fig. 18b, Base 1 = guanine, Base
5 of the next. A third nucleotide can become joined at 2 = thymine, Base 3 = adenine, and Base n = cytosine, the
carbon 3 of the right-hand sugar ring, and the process can structure would be represented as GTA ... C.
continue to give a chain of several linked nucleotides—an The two polynucleotide chains in DNA molecules are
oligonucleotide—or a chain of hundreds or even thou- believed to be wound around each other to give a regular
sands of covalently bonded nucleotides, a polynucleotide secondary structure, a right-handed double helix (Fig. 19).
(Fig. 18b). Most DNA molecules consist of two polynu- The two chains run in opposite directions with the phos-
cleotide chains, while RNA molecules usually contain phate and sugar groups on the outside of the helix and
only one polynucleotide chain. the bases in the interior. The proposal for this structure
At one end of a chain the group attached to carbon 5 of was first made by Watson and Crick and led to their be-
the sugar ring is not involved in a phosphodiester link. This ing awarded the Nobel Prize in 1962. Since the bases are
is referred to as the 5 end of the chain and by convention flat, they can stack on top of one another in any sequence
is written at the left end of the chain. At the other end, almost at right angles to the helix axis. The two chains of