Page 44 - Academic Press Encyclopedia of Physical Science and Technology 3rd Polymer
P. 44
P1: GQT/LBX P2: GQT/MBQ QC: FYD Final Pages
Encyclopedia of Physical Science and Technology EN008C-602 July 25, 2001 20:31
Macromolecules, Structure 859
first entirely synthetic resin—the phenol-formaldehyde The structure of a macromolecule—on chemical, mi-
condensation product known as Bakelite—between 1905 crostructural,conformational,andmorphologicallevels—
and 1909. However, it is unlikely that any of the devel- has a vital relationship to the properties of the material.
opers of these materials recognized their true chemical Because the properties of a material dictate its ultimate
nature as macromolecules. Even at a time—the period use, it is very important to obtain a fundamental under-
of approximately 1900 to 1930—when organic chemistry standing of the structure of macromolecules.
was achieving an advanced state of development, the idea Although this article will deal mainly with macro-
of covalently bonded molecules with molecular weights molecules of the synthetic kind, essentially all methods of
of 100,000 or even higher was not accepted. It was felt structural characterization described here pertain equally
that such structures would be inherently unstable. Instead, well to biological polymers.Before specifying thedetailed
it was commonly assumed that substances with polymeric chemical structure of the monomer units, we can describe
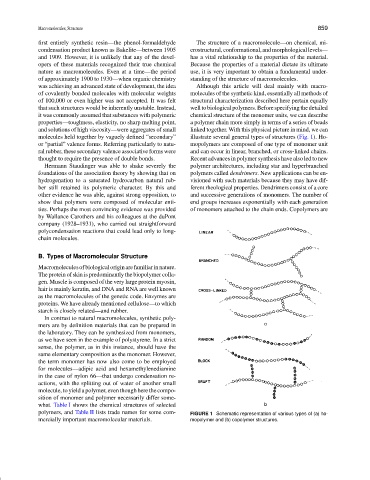
properties—toughness, elasticity, no sharp melting point, a polymer chain more simply in terms of a series of beads
and solutions of high viscosity—were aggregates of small linked together. With this physical picture in mind, we can
molecules held together by vaguely defined “secondary” illustrate several general types of structures (Fig. 1). Ho-
or “partial” valence forms. Referring particularly to natu- mopolymers are composed of one type of monomer unit
ral rubber, these secondary valence associative forms were and can occur in linear, branched, or cross-linked chains.
thought to require the presence of double bonds. Recent advances in polymer synthesis have also led to new
Hermann Staudinger was able to shake severely the polymer architectures, including star and hyperbranched
foundations of the association theory by showing that on polymers called dendrimers. New applications can be en-
hydrogenation to a saturated hydrocarbon natural rub- visioned with such materials because they may have dif-
ber still retained its polymeric character. By this and ferent rheological properties. Dendrimers consist of a core
other evidence he was able, against strong opposition, to and successive generations of monomers. The number of
show that polymers were composed of molecular enti- end groups increases exponentially with each generation
ties. Perhaps the most convincing evidence was provided of monomers attached to the chain ends. Copolymers are
by Wallance Carothers and his colleagues at the duPont
company (1928–1931), who carried out straightforward
polycondensation reactions that could lead only to long-
chain molecules.
B. Types of Macromolecular Structure
Macromoleculesofbiologicaloriginarefamiliarinnature.
The protein of skin is predominantly the biopolymer colla-
gen. Muscle is composed of the very large protein myosin,
hair is mainly keratin, and DNA and RNA are well known
as the macromolecules of the genetic code. Enzymes are
proteins. We have already mentioned cellulose—to which
starch is closely related—and rubber.
In contrast to natural macromolecules, synthetic poly-
mers are by definition materials that can be prepared in
the laboratory. They can be synthesized from monomers,
as we have seen in the example of polystyrene. In a strict
sense, the polymer, as in this instance, should have the
same elementary composition as the monomer. However,
the term monomer has now also come to be employed
for molecules—adipic acid and hexamethylenediamine
in the case of nylon 66—that undergo condensation re-
actions, with the splitting out of water of another small
molecule, to yield a polymer, even though here the compo-
sition of monomer and polymer necessarily differ some-
what. Table I shows the chemical structures of selected
polymers, and Table II lists trade names for some com- FIGURE 1 Schematic representation of various types of (a) ho-
mercially important macromolecular materials. mopolymer and (b) copolymer structures.