Page 48 - Academic Press Encyclopedia of Physical Science and Technology 3rd Polymer
P. 48
P1: GQT/LBX P2: GQT/MBQ QC: FYD Final Pages
Encyclopedia of Physical Science and Technology EN008C-602 July 25, 2001 20:31
Macromolecules, Structure 863
TABLE II Trade Names of Selected Polymers
ABS Acrylonitrile–butadiene–styrene graft copolymers
Acrylic Poly(acrylonitrile)
Amberlite Ion-exchange resins
Bakelite Phenol-formaldehyde
Buna N Butadiene–acrylonitrile copolymer
Buna S (SBR) Butadiene–styrene copolymer
Butyl rubber Poly(isobutylene) + ∼1% isoprene
Carbowax Poly(ethylene glycol)
Cellophane Cellulose hydrate
Celluloid Cellulose nitrate
Cycolac ABS
Dacron Poly(ethylene terephthalate) fiber
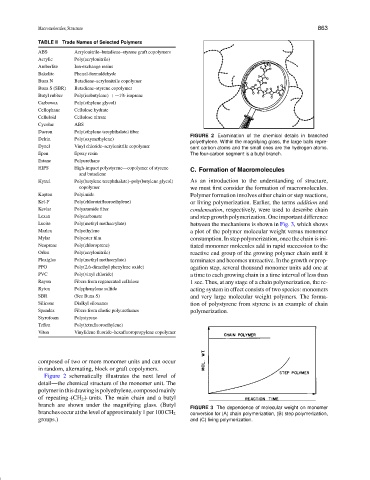
FIGURE 2 Examination of the chemical details in branched
Delrin Poly(oxymethylene)
polyethylene. Within the magnifying glass, the large balls repre-
Dynel Vinyl chloride–acrylonitrile copolymer sent carbon atoms and the small ones are the hydrogen atoms.
Epon Epoxy resin The four-carbon segment is a butyl branch.
Estane Polyurethane
HIPS High-impact polystyrene—copolymer of styrene C. Formation of Macromolecules
and butadiene
Hytrel Poly(butylene terephthalate)–poly(butylene glycol) As an introduction to the understanding of structure,
copolymer we must first consider the formation of macromolecules.
Kapton Polyimide Polymer formation involves either chain or step reactions,
Kel-F Poly(chlorotrifluoroethylene) or living polymerization. Earlier, the terms addition and
Kevlar Polyaramide fiber condensation, respectively, were used to describe chain
Lexan Polycarbonate and step growth polymerization. One important difference
Lucite Poly(methyl methacrylate) between the mechanisms is shown in Fig. 3, which shows
Marlex Polyethylene a plot of the polymer molecular weight versus monomer
Mylar Polyester film consumption. In step polymerization, once the chain is ini-
Neoprene Poly(chloroprene) tiated monomer molecules add in rapid succession to the
Orlon Poly(acrylonitrile) reactive end group of the growing polymer chain until it
Plexiglas Poly(methyl methacrylate) terminates and becomes unreactive. In the growth or prop-
PPO Poly(2,6-dimethyl phenylene oxide) agation step, several thousand monomer units add one at
PVC Poly(vinyl chloride) a time to each growing chain in a time interval of less than
Rayon Fibers from regenerated cellulose 1 sec. Thus, at any stage of a chain polymerization, the re-
Ryton Polyphenylene sulfide acting system in effect consists of two species: monomers
SBR (See Buna S) and very large molecular weight polymers. The forma-
Silicone Dialkyl siloxanes tion of polystyrene from styrene is an example of chain
Spandex Fibers from elastic polyurethanes polymerization.
Styrofoam Polystyrene
Teflon Poly(tetrafluoroethylene)
Viton Vinylidene fluoride–hexafluoropropylene copolymer
composed of two or more monomer units and can occur
in random, alternating, block or graft copolymers.
Figure 2 schematically illustrates the next level of
detail—the chemical structure of the monomer unit. The
polymer in this drawing is polyethylene, composed mainly
of repeating (CH 2 ) units. The main chain and a butyl
branch are shown under the magnifying glass. (Butyl
FIGURE 3 The dependence of molecular weight on monomer
branches occur at the level of approximately 1 per 100 CH 2 conversion for (A) chain polymerization, (B) step polymerization,
groups.) and (C) living polymerization.