Page 50 - Academic Press Encyclopedia of Physical Science and Technology 3rd Polymer
P. 50
P1: GQT/LBX P2: GQT/MBQ QC: FYD Final Pages
Encyclopedia of Physical Science and Technology EN008C-602 July 25, 2001 20:31
Macromolecules, Structure 865
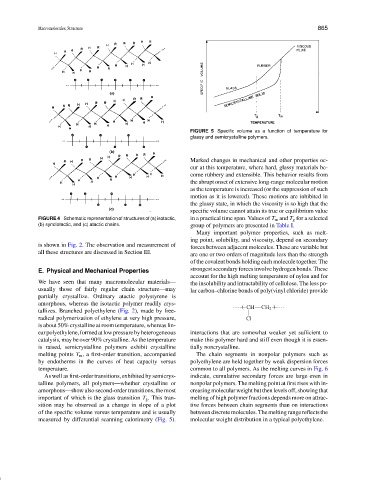
FIGURE 5 Specific volume as a function of temperature for
glassy and semicrystalline polymers.
Marked changes in mechanical and other properties oc-
cur at this temperature, where hard, glassy materials be-
come rubbery and extensible. This behavior results from
the abrupt onset of extensive long-range molecular motion
as the temperature is increased (or the suppression of such
motion as it is lowered). These motions are inhibited in
the glassy state, in which the viscosity is so high that the
specific volume cannot attain its true or equilibrium value
FIGURE 4 Schematic representation of structures of (a) isotactic, in a practical time span. Values of T m and T g for a selected
(b) syndiotactic, and (c) atactic chains. group of polymers are presented in Table I.
Many important polymer properties, such as melt-
ing point, solubility, and viscosity, depend on secondary
is shown in Fig. 2. The observation and measurement of
forces between adjacent molecules. These are variable but
all these structures are discussed in Section III.
are one or two orders of magnitude less than the strength
of the covalent bonds holding each molecule together. The
E. Physical and Mechanical Properties strongest secondary forces involve hydrogen bonds. These
account for the high melting temperature of nylon and for
We have seen that many macromolecular materials— the insolubility and intractability of cellulose. The less po-
usually those of fairly regular chain structure—may lar carbon–chlorine bonds of poly(vinyl chloride) provide
partially crystallize. Ordinary atactic polystyrene is
amorphous, whereas the isotactic polymer readily crys-
( CH CH 2 (
tallizes. Branched polyethylene (Fig. 2), made by free-
radical polymerization of ethylene at very high pressure, Cl
is about 50% crystalline at room temperature, whereas lin-
earpolyethylene,formedatlowpressurebyheterogeneous interactions that are somewhat weaker yet sufficient to
catalysis, may be over 90% crystalline. As the temperature make this polymer hard and stiff even though it is essen-
is raised, semicrystalline polymers exhibit crystalline tially noncrystalline.
melting points T m ,a first-order transition, accompanied The chain segments in nonpolar polymers such as
by endotherms in the curves of heat capacity versus polyethylene are held together by weak dispersion forces
temperature. common to all polymers. As the melting curves in Fig. 6
As well as first-order transitions, exhibited by semicrys- indicate, cumulative secondary forces are large even in
talline polymers, all polymers—whether crystalline or nonpolar polymers. The melting point at first rises with in-
amorphous—show also second-order transitions, the most creasing molecular weight but then levels off, showing that
important of which is the glass transition T g . This tran- melting of high polymer fractions depends more on attrac-
sition may be observed as a change in slope of a plot tive forces between chain segments than on interactions
of the specific volume versus temperature and is usually between discrete molecules. The melting range reflects the
measured by differential scanning calorimetry (Fig. 5). molecular weight distribution in a typical polyethylene.