Page 124 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 124
P1: GLM Final Pages
Encyclopedia of Physical Science and Technology EN007C-307 June 29, 2001 19:40
Halogen Chemistry 201
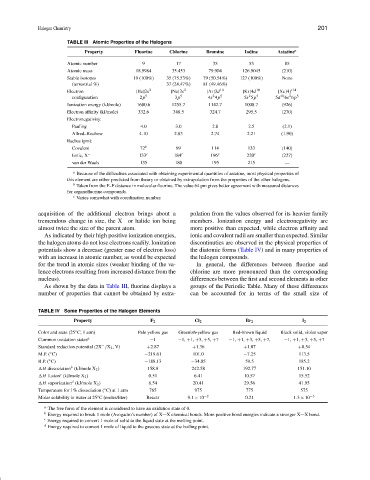
TABLE III Atomic Properties of the Halogens
Property Fluorine Chlorine Bromine Iodine Astatine a
Atomic number 9 17 35 53 85
Atomic mass 18.9984 35.453 79.904 126.9045 (210)
Stable isotopes 19 (100%) 35 (75.53%) 79 (50.54%) 127 (100%) None
(terrestrial %) 37 (24.47%) 81 (49.46%)
Electron [He]2s 2 [Ne]3s 2 [Ar]3d 10 [Kr]4d 10 [Xe]4 f 14
2
2
2
10
configuration 2p 5 3p 5 4s 4p 5 5s 5p 5 5d 6s 6p 5
Ionization energy (kJ/mole) 1680.6 1255.7 1142.7 1008.7 (926)
Electron affinity (kJ/mole) 332.6 348.5 324.7 295.5 (270)
Electronegativity:
Pauling 4.0 3.0 2.8 2.5 (2.1)
Allred–Rochow 4.10 2.83 2.74 2.21 (1.90)
Radius (pm):
Covalent 72 b 99 114 133 (140)
Ionic, X − 133 c 184 c 196 c 220 c (227)
van der Waals 135 180 195 215 —
a Because of the difficulties associated with obtaining experimental quantities of astatine, most physical properties of
this element are either predicted from theory or obtained by extrapolation from the properties of the other halogens.
b Taken from the F–F distance in molecular fluorine. The value 64 pm gives better agreement with measured distances
for organofluorine compounds.
c Varies somewhat with coordination number.
acquisition of the additional electron brings about a polation from the values observed for its heavier family
−
tremendous change in size, the X or halide ion being members. Ionization energy and electronegativity are
almost twice the size of the parent atom. more positive than expected, while electron affinity and
As indicated by their high positive ionization energies, ionic and covalent radii are smaller than expected. Similar
the halogen atoms do not lose electrons readily. Ionization discontinuties are observed in the physical properties of
potentials show a decrease (greater ease of electron loss) the diatomic forms (Table IV) and in many properties of
with an increase in atomic number, as would be expected the halogen compounds.
for the trend in atomic sizes (weaker binding of the va- In general, the differences between fluorine and
lence electrons resulting from increased distance from the chlorine are more pronounced than the corresponding
nucleus). differences between the first and second elements in other
As shown by the data in Table III, fluorine displays a groups of the Periodic Table. Many of these differences
number of properties that cannot be obtained by extra- can be accounted for in terms of the small size of
TABLE IV Some Properties of the Halogen Elements
Property F 2 Cl 2 Br 2 I 2
Color and state (25 C, 1 atm) Pale yellow gas Greenish-yellow gas Red-brown liquid Black solid, violet vapor
◦
Common oxidation states a −1 −1, +1, +3, +5, +7 −1, +1, +3, +5, +7, −1, +1, +3, +5, +7
Standard reduction potential (2X /X 2 , V) +2.87 +1.36 +1.07 +0.54
−
◦
M.P. ( C) −219.61 101.0 −7.25 113.5
B.P. ( C) −188.13 −34.05 59.5 185.2
◦
b
H dissociation (kJ/mole X 2 ) 158.8 242.58 192.77 151.10
c
H fusion (kJ/mole X 2 ) 0.51 6.41 10.57 15.52
d
H vaporization (kJ/mole X 2 ) 6.54 20.41 29.56 41.95
◦
Temperature for 1% dissociation ( C) at 1 atm 765 975 775 575
Molar solubility in water at 25 C (moles/liter) Reacts 9.1 × 10 −2 0.21 1.3 × 10 −3
◦
a The free form of the element is considered to have an oxidation state of 0.
b Energy required to break 1 mole (Avogadro’s number) of X X chemical bonds. More positive bond energies indicate a stronger X X bond.
c Energy required to convert 1 mole of solid to the liquid state at the melting point.
d Energy required to convert 1 mole of liquid to the gaseous state at the boiling point.