Page 128 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 128
P1: GLM Final Pages
Encyclopedia of Physical Science and Technology EN007C-307 June 29, 2001 19:40
Halogen Chemistry 205
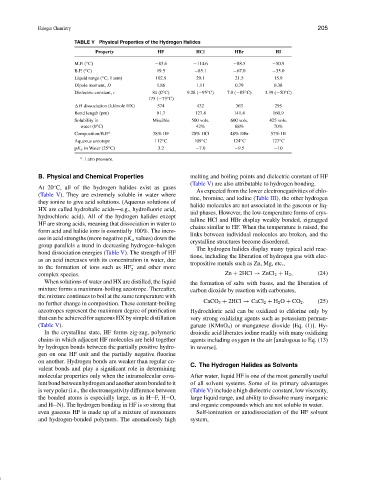
TABLE V Physical Properties of the Hydrogen Halides
Property HF HCl HBr HI
M.P. ( C) −83.6 −114.6 −88.5 −50.9
◦
◦
B.P. ( C) 19.5 −85.1 −67.0 −35.0
Liquid range ( C, 1 atm) 102.9 29.1 21.5 15.9
◦
Dipole moment, D 1.86 1.11 0.79 0.38
◦
Dielectric constant, 84 (0 C) 9.28 (−95 C) 7.0 (−85 C) 3.39 (−50 C)
◦
◦
◦
175 (−73 C)
◦
H dissociation (kJ/mole HX) 574 432 363 295
Bond length (pm) 91.7 127.4 141.4 160.9
Solubility in Miscible 500 vols. 600 vols. 425 vols.
◦
water (0 C) 42% 68% 70%
Composition/B.P. a 38% HF 20% HCl 48% HBr 57% HI
◦
Aqueous azeotope 112 C 109 C 124 C 127 C
◦
◦
◦
◦
pK a in Water (25 C) 3.2 −7.0 −9.5 −10
a 1 atm pressure.
B. Physical and Chemical Properties melting and boiling points and dielectric constant of HF
(Table V) are also attributable to hydrogen bonding.
At 20 C, all of the hydrogen halides exist as gases
◦
As expected from the lower electronegativities of chlo-
(Table V). They are extremely soluble in water where
rine, bromine, and iodine (Table III), the other hydrogen
they ionize to give acid solutions. (Aqueous solutions of
halide molecules are not associated in the gaseous or liq-
HX are called hydrohalic acids—e.g., hydrofluoric acid,
uid phases. However, the low-temperature forms of crys-
hydrochloric acid). All of the hydrogen halides except
talline HCl and HBr display weakly bonded, zigzagged
HF are strong acids, meaning that dissociation in water to
chains similar to HF. When the temperature is raised, the
form acid and halide ions is essentially 100%. The incre-
links between individual molecules are broken, and the
ase in acid strengths (more negative pK a values) down the
crystalline structures become disordered.
group parallels a trend in decreasing hydrogen–halogen
The hydrogen halides display many typical acid reac-
bond dissociation energies (Table V). The strength of HF
tions, including the liberation of hydrogen gas with elec-
as an acid increases with its concentration in water, due
tropositive metals such as Zn, Mg, etc.,
to the formation of ions such as HF − and other more
2
complex species. Zn + 2HCl → ZnCl 2 + H 2 , (24)
When solutions of water and HX are distilled, the liquid the formation of salts with bases, and the liberation of
mixture forms a maximum-boiling azeotrope. Thereafter, carbon dioxide by reaction with carbonates,
the mixture continues to boil at the same temperature with
CaCO 3 + 2HCl → CaCl 2 + H 2 O + CO 2 . (25)
no further change in composition. These constant-boiling
azeotropes represent the maximum degree of purification Hydrochloric acid can be oxidized to chlorine only by
that can be achieved for aqueous HX by simple distillation very strong oxidizing agents such as potassium perman-
(Table V). ganate (KMnO 4 ) or manganese dioxide [Eq. (1)]. Hy-
In the crystalline state, HF forms zig-zag, polymeric droiodic acid liberates iodine readily with many oxidizing
chains in which adjacent HF molecules are held together agents including oxygen in the air [analogous to Eq. (13)
by hydrogen bonds between the partially positive hydro- in reverse].
gen on one HF unit and the partially negative fluorine
on another. Hydrogen bonds are weaker than regular co- C. The Hydrogen Halides as Solvents
valent bonds and play a significant role in determining
molecular properties only when the intramolecular cova- After water, liquid HF is one of the most generally useful
lent bond between hydrogen and another atom bonded to it of all solvent systems. Some of its primary advantages
is very polar (i.e., the electronegativity difference between (Table V) include a high dielectric constant, low viscosity,
the bonded atoms is especially large, as in H F, H O, large liquid range, and ability to dissolve many inorganic
and H N). The hydrogen bonding in HF is so strong that and organic compounds which are not soluble in water.
even gaseous HF is made up of a mixture of monomers Self-ionization or autodissociation of the HF solvent
and hydrogen-bonded polymers. The anomalously high system,