Page 169 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 169
P1: GPA Final Pages
Encyclopedia of Physical Science and Technology EN007D-343 July 10, 2001 20:13
Inorganic Exotic Molecules 827
phous and is readily obtained by heating P 4 up to 400 Cin
◦
a sealed container. Red phosphorus consists of polymers
obtained by partial opening and conjoining (or catenation)
of the P 4 tetrahedra.
When white phosphorus is heated in organic solvents in
the presence of certain cyclopentadienyl transition metal
carbonylcomplexes,newcomplexescontainingthemono-
cyclic P 6 analogue of benzene are found (Fig. 2). There
are also a few compounds having metal atoms on both
sides of a P 5 ring that can be recognized as the analogue
−
of the cyclopentadienyl anion, [C 5 H 5 ] .
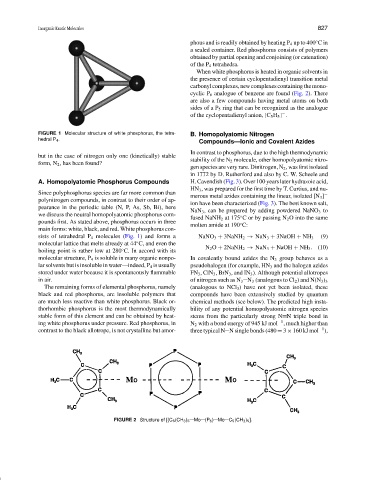
FIGURE 1 Molecular structure of white phosphorus, the tetra- B. Homopolyatomic Nitrogen
hedral P 4 .
Compounds—Ionic and Covalent Azides
In contrast to phosphorus, due to the high thermodynamic
but in the case of nitrogen only one (kinetically) stable
stability of the N 2 molecule, other homopolyatomic nitro-
form, N 2 , has been found?
gen species are very rare. Dinitrogen, N 2 ,was first isolated
in 1772 by D. Rutherford and also by C. W. Scheele and
A. Homopolyatomic Phosphorus Compounds H. Cavendish (Fig. 3). Over 100 years later hydrazoic acid,
HN 3 , was prepared for the first time by T. Curtius, and nu-
Since polyphosphorus species are far more common than
merous metal azides containing the linear, isolated [N 3 ] −
polynitrogen compounds, in contrast to their order of ap-
ion have been characterized (Fig. 3). The best known salt,
pearance in the periodic table (N, P, As, Sb, Bi), here
NaN 3 , can be prepared by adding powdered NaNO 3 to
we discuss the neutral homopolyatomic phosphorus com-
fused NaNH 2 at 175 C or by passing N 2 O into the same
◦
pounds first. As stated above, phosphorus occurs in three
molten amide at 190 C:
◦
main forms: white, black, and red. White phosphorus con-
sists of tetrahedral P 4 molecules (Fig. 1) and forms a NaNO 3 + 3NaNH 2 → NaN 3 + 3NaOH + NH 3 (9)
molecular lattice that melts already at 44 C, and even the
◦
N 2 O + 2NaNH 2 → NaN 3 + NaOH + NH 3 . (10)
boiling point is rather low at 280 C. In accord with its
◦
molecular structure, P 4 is soluble in many organic nonpo- In covalently bound azides the N 3 group behaves as a
lar solvents but is insoluble in water—indeed, P 4 is usually pseudohalogen (for example, HN 3 and the halogen azides
stored under water because it is spontaneously flammable FN 3 , ClN 3 , BrN 3 , and IN 3 ). Although potential allotropes
in air. of nitrogen such as N 3 N 3 (analogous to Cl 2 ) and N(N 3 ) 3
The remaining forms of elemental phosphorus, namely (analogous to NCl 3 ) have not yet been isolated, these
black and red phosphorus, are insoluble polymers that compounds have been extensively studied by quantum
are much less reactive than white phosphorus. Black or- chemical methods (see below). The predicted high insta-
thorhombic phosphorus is the most thermodynamically bility of any potential homopolyatomic nitrogen species
stable form of this element and can be obtained by heat- stems from the particularly strong N N triple bond in
−1
ing white phosphorus under pressure. Red phosphorus, in N 2 with a bond energy of 945 kJ mol , much higher than
−1
contrast to the black allotrope, is not crystalline but amor- three typical N N single bonds (480 = 3 × 160 kJ mol ),
FIGURE 2 Structure of [(C 5 (CH 3 ) 5 Mo (P 6 ) Mo C 5 (CH 3 ) 5 ].