Page 236 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 236
P1: GNS/GUB P2: GQT Final Pages
Encyclopedia of Physical Science and Technology EN009I-110 July 18, 2001 0:40
408 Metal Cluster Chemistry
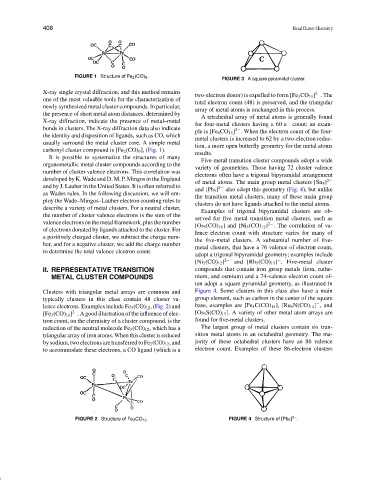
FIGURE 1 Structure of Fe 2 (CO) 9 .
FIGURE 3 A square pyramidal cluster.
X-ray single crystal diffraction, and this method remains 2 −
two-electron donor) is expelled to form [Fe 3 CO 11 ] . The
one of the most valuable tools for the characterization of
total electron count (48) is preserved, and the triangular
newly synthesized metal cluster compounds. In particular,
array of metal atoms is unchanged in this process.
the presence of short metal atom distances, determined by
A tetrahedral array of metal atoms is generally found
X-ray diffraction, indicate the presence of metal–metal
for four-metal clusters having a 60 e count; an exam-
−
bonds in clusters. The X-ray diffraction data also indicate
ple is [Fe 4 CO 13 ] . When the electron count of the four-
2−
the identity and disposition of ligands, such as CO, which
metal clusters is increased to 62 by a two-electron reduc-
usually surround the metal cluster core. A simple metal
tion, a more open butterfly geometry for the metal atoms
carbonyl cluster compound is [Fe 2 (CO) 9 ], (Fig. 1).
results.
It is possible to systematize the structures of many
Five-metal transition cluster compounds adopt a wide
organometallic metal cluster compounds according to the
variety of geometries. Those having 72 cluster valence
number of cluster valence electrons. This correlation was
electrons often have a trigonal bipyramidal arrangement
developed by K. Wade and D. M. P. Mingos in the England
of metal atoms. The main group metal clusters [Sn 5 ] 2−
and by J. Lauher in the United States. It is often referred to 2−
and [Pb 5 ] also adopt this geometry (Fig. 4), but unlike
as Wades rules. In the following discussion, we will em-
the transition metal clusters, many of these main group
ploy the Wade–Mingos–Lauher electron counting rules to
clusters do not have ligands attached to the metal atoms.
describe a variety of metal clusters. For a neutral cluster,
Examples of trigonal bipyramidal clusters are ob-
the number of cluster valence electrons is the sum of the
served for five metal transition metal clusters, such as
valenceelectronsonthemetalframework,plusthenumber
[Os 5 (CO) 16 ] and [Ni 5 (CO) 12 ] . The correlation of va-
2−
of electrons donated by ligands attached to the cluster. For
lence electron count with structure varies for many of
a positively charged cluster, we subtract the charge num-
the five-metal clusters. A substantial number of five-
ber, and for a negative cluster, we add the charge number
metal clusters, that have a 76 valence of electron count,
to determine the total valence electron count.
adopt a trigonal bipyramidal geometry; examples include
−
[Ni 5 (CO) 12 ] 2− and [Rh 5 (CO) 15 ] . Five-metal cluster
II. REPRESENTATIVE TRANSITION compounds that contain iron group metals (iron, ruthe-
METAL CLUSTER COMPOUNDS nium, and osmium) and a 74-valence electron count of-
ten adopt a square pyramidal geometry, as illustrated in
Clusters with triangular metal arrays are common and Figure 4. Some clusters in this class also have a main
typically clusters in this class contain 48 cluster va- group element, such as carbon in the center of the square
−
lence electrons. Examples include Fe 3 (CO) 12 , (Fig. 2) and base, examples are [Fe 5 C(CO) 15 ], [Ru 5 N(CO) 14 ] , and
−
2
[Fe 3 (CO) 11 ] . A good illustration of the influence of elec- [Os 5 S(CO) 15 ]. A variety of other metal atom arrays are
tron count, on the chemistry of a cluster compound, is the found for five-metal clusters.
reduction of the neutral molecule Fe 3 (CO) 12 , which has a The largest group of metal clusters contain six tran-
triangular array of iron atoms. When this cluster is reduced sition metal atoms in an octahedral geometry. The ma-
by sodium, two electrons are transferred to Fe 3 (CO) 12 , and jority of these octahedral clusters have an 86 valence
to accommodate these electrons, a CO ligand (which is a electron count. Examples of these 86-electron clusters
FIGURE 2 Structure of Fe 3 CO 12 . FIGURE 4 Structure of [Pb 5 ] 2− .