Page 231 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 231
P1: GPB Final Pages
Encyclopedia of Physical Science and Technology EN009I-420 July 10, 2001 15:8
378 Mesoporous Materials, Synthesis and Properties
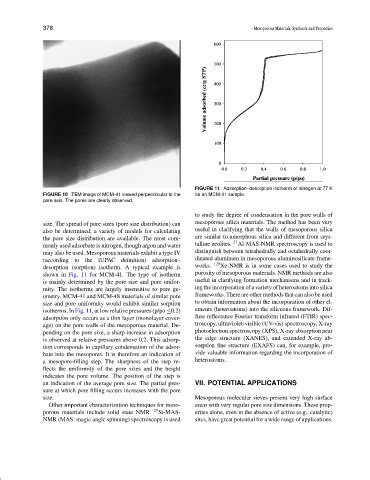
FIGURE 11 Adsorption–desorption isotherm of nitrogen at 77 K
FIGURE 10 TEM image of MCM-41 viewed perpendicular to the on an MCM-41 sample.
pore axis. The pores are clearly observed.
to study the degree of condensation in the pore walls of
size. The spread of pore sizes (pore size distribution) can mesoporous silica materials. The method has been very
also be determined; a variety of models for calculating useful in clarifying that the walls of mesoporous silica
the pore size distribution are available. The most com- are similar to amorphous silica and different from crys-
27
monly used adsorbate is nitrogen, though argon and water talline zeolites. Al-MAS-NMR spectroscopy is used to
may also be used. Mesoporous materials exhibit a type IV distinguish between tetrahedrally and octahedrally coor-
(according to the IUPAC definition) adsorption– dinated aluminum in mesoporous aluminosilicate frame-
129
desorption (sorption) isotherm. A typical example is works. Xe-NMR is in some cases used to study the
shown in Fig. 11 for MCM-41. The type of isotherm porosity of mesoporous materials. NMR methods are also
is mainly determined by the pore size and pore unifor- useful in clarifying formation mechanisms and in track-
mity. The isotherms are largely insensitive to pore ge- ing the incorporation of a variety of heteroatoms into silica
ometry. MCM-41 and MCM-48 materials of similar pore frameworks. There are other methods that can also be used
size and pore uniformity would exhibit similar sorption to obtain information about the incorporation of other el-
isotherms. In Fig. 11, at low relative pressures (p/po ≤0.2) ements (heteroatoms) into the siliceous framework. Dif-
adsorption only occurs as a thin layer (monolayer cover- fuse reflectance Fourier transform infrared (FTIR) spec-
age) on the pore walls of the mesoporous material. De- troscopy, ultraviolet-visible (UV-vis) spectroscopy, X-ray
pending on the pore size, a sharp increase in adsorption photoelectron spectroscopy (XPS), X-ray absorption near
is observed at relative pressures above 0.2. This adsorp- the edge structure (XANES), and extended X-ray ab-
tion corresponds to capillary condensation of the adsor- sorption fine structure (EXAFS) can, for example, pro-
bate into the mesopores. It is therefore an indication of vide valuable information regarding the incorporation of
a mesopore-filling step. The sharpness of the step re- heteroatoms.
flects the uniformity of the pore sizes and the height
indicates the pore volume. The position of the step is
an indication of the average pore size. The partial pres- VII. POTENTIAL APPLICATIONS
sure at which pore filling occurs increases with the pore
size. Mesoporous molecular sieves present very high surface
Other important characterization techniques for meso- areas with very regular pore size dimensions. These prop-
porous materials include solid state NMR. 29 Si-MAS- erties alone, even in the absence of active (e.g., catalytic)
NMR (MAS: magic angle spinning) spectroscopy is used sites, have great potential for a wide range of applications.