Page 269 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 269
P1: GNH Final Pages
Encyclopedia of Physical Science and Technology EN009M-428 July 18, 2001 1:6
526 Metal Particles and Cluster Compounds
properties,heatcapacity,andmagneticsusceptibilities,for deal of evidence available indicating that higher reaction
example, may be very different for small particles relative rates, more complete conversions, and varying reaction
to the bulk due to the increase in S. This would be ex- selectivities are possible using small metal particles (as
pected to be especially relevant at low temperature where compared with bulk metals). However, sorting out surface
δ kT . area effects, support effects, oxidation state changes, and
Defects in small particles have been considered by the- the behavior of defects has caused a great deal of confu-
orists. Density of states at the Fermi level in 13-atom clus- sion and inherent reactivities of metal atoms versus bulk
ters of Fe, Ni, and Cu showed the highest densities in Fe. metal have been difficult to ascertain. However, it has been
The d bandwidth for the case of a 13-atom Ni particle learned that certain chemical reactions are more sensitive
was much smaller than that of the bulk. The model used to surface structure than others. These reactions are (1)
in these calculations was varied to include special surface C C bond breaking (hydrogenolysis and skeletal isomer-
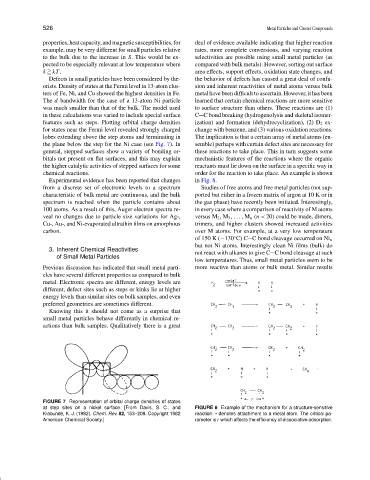
features such as steps. Plotting orbital charge densities ization) and formation (dehydrocyclization), (2) D 2 ex-
for states near the Fermi level revealed strongly charged change with benzene, and (3) various oxidation reactions.
lobes extending above the step atoms and terminating in The implication is that a certain array of metal atoms (en-
the plane below the step for the Ni case (see Fig. 7). In semble) perhaps with certain defect sites are necessary for
general, stepped surfaces show a variety of bonding or- these reactions to take place. This in turn suggests some
bitals not present on flat surfaces, and this may explain mechanistic features of the reactions where the organic
the higher catalytic activities of stepped surfaces for some reactants must lie down on the surface in a specific way in
chemical reactions. order for the reaction to take place. An example is shown
Experimental evidence has been reported that changes in Fig. 8.
from a discrete set of electronic levels to a spectrum Studies of free atoms and free metal particles (not sup-
characteristic of bulk metal are continuous, and the bulk ported but either in a frozen matrix of argon at 10 K or in
spectrum is reached when the particle contains about the gas phase) have recently been initiated. Interestingly,
100 atoms. As a result of this, Auger electron spectra re- in every case where a comparison of reactivity of M atoms
veal no changes due to particle size variations for Ag-, versus M 2 , M 3 ,..., M n (n < 20) could be made, dimers,
Cu-, Au-, and Ni-evaporated ultrathin films on amorphous trimers, and higher clusters showed increased activities
carbon. over M atoms. For example, at a very low temperature
◦
of 150 K (−130 C) C C bond cleavage occurred on Ni n
but not Ni atoms. Interestingly clean Ni films (bulk) do
3. Inherent Chemical Reactivities
not react with alkanes to give C C bond cleavage at such
of Small Metal Particles
low temperatures. Thus, small metal particles seem to be
Previous discussion has indicated that small metal parti- more reactive than atoms or bulk metal. Similar results
cles have several different properties as compared to bulk
metal. Electronic spectra are different, energy levels are
different, defect sites such as steps or kinks lie at higher
energy levels than similar sites on bulk samples, and even
preferred geometries are sometimes different.
Knowing this it should not come as a surprise that
small metal particles behave differently in chemical re-
actions than bulk samples. Qualitatively there is a great
FIGURE 7 Representation of orbital charge densities of states
at step sites on a nickel surface. [From Davis, S. C., and FIGURE 8 Example of the mechanism for a structure-sensitive
Klabunde, K. J. (1982). Chem. Rev. 82, 153–208. Copyright 1982 reaction: * denotes attachment to a metal atom. The critical pa-
American Chemical Society.] rameter is r which affects the efficiency of dissociative adsorption.