Page 264 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 264
P1: GNH Final Pages
Encyclopedia of Physical Science and Technology EN009M-428 July 18, 2001 1:6
Metal Particles and Cluster Compounds 521
structures. These deformations involve the transforma- d band. As with the pair potential calculations the energy
tion of sites of tetrahedral symmetry to sites of octahedral does not vary strongly with geometry and many structures
symmetry. are calculated to be nearly isoenergetic.
In addition to the problem represented by the fact For metal clusters, it is now possible, through first
that icosahedra (tetrahedra) cannot form extended three- principle theoretical (calculational) approaches, to predict
dimensional structures, the Mackay icosahedra do not and better understand vibrational spectra, optical band
grow smoothly from one into another by simply placing gaps, polarizability, quantum confinement, and structural
new atoms in the centers of each triangular face. Such predictions. One modern approach is to use pseudopoten-
a growth pattern by the formation of caps might be ex- tial density functional methods (PDFM), in particular to
pected to be energy efficient and does lead to other magic predict optical and dielectric properties. Similarly, using
numbers which are experimentally seen. For the larger molecular dynamics simulations, it is possible to create
particles a highly symmetrical geometry was assumed models for cluster structures. This has been especially
and the evolution, if any, of the particle with time was valuable for predicting a three-dimensional image for
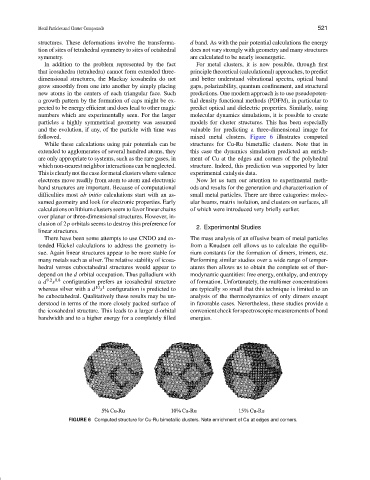
followed. mixed metal clusters. Figure 6 illustrates computed
While these calculations using pair potentials can be structures for Cu-Ru bimetallic clusters. Note that in
extended to agglomerates of several hundred atoms, they this case the dynamics simulation predicted an enrich-
are only appropriate to systems, such as the rare gases, in ment of Cu at the edges and corners of the polyhedral
which non-nearest neighbor interactions can be neglected. structure. Indeed, this prediction was supported by later
Thisisclearlynotthecaseformetalclusterswherevalence experimental catalysis data.
electrons move readily from atom to atom and electronic Now let us turn our attention to experimental meth-
band structures are important. Because of computational ods and results for the generation and characterization of
difficulties most ab initio calculations start with an as- small metal particles. There are three catagories: molec-
sumed geometry and look for electronic properties. Early ular beams, matrix isolation, and clusters on surfaces, all
calculations on lithium clusters seem to favor linear chains of which were introduced very briefly earlier.
over planar or three-dimensional structures. However, in-
clusion of 2p orbitals seems to destroy this preference for
2. Experimental Studies
linear structures.
There have been some attempts to use CNDO and ex- The mass analysis of an effusive beam of metal particles
tended H¨uckel calculations to address the geometry is- from a Knudsen cell allows us to calculate the equilib-
sue. Again linear structures appear to be more stable for rium constants for the formation of dimers, trimers, etc.
many metals such as silver. The relative stability of icosa- Performing similar studies over a wide range of temper-
hedral versus cuboctahedral structures would appear to atures then allows us to obtain the complete set of ther-
depend on the d orbital occupation. Thus palladium with modynamic quantities: free energy, enthalpy, and entropy
a d 9.2 0.8 configuration prefers an icosahedral structure of formation. Unfortunately, the multimer concentrations
s
10 1
whereas silver with a d s configuration is predicted to are typically so small that this technique is limited to an
be cuboctahedral. Qualitatively these results may be un- analysis of the thermodynamics of only dimers except
derstood in terms of the more closely packed surface of in favorable cases. Nevertheless, these studies provide a
the icosahedral structure. This leads to a larger d-orbital convenient check for spectroscopic measurements of bond
bandwidth and to a higher energy for a completely filled energies.
FIGURE 6 Computed structure for Cu-Ru bimetallic clusters. Note enrichment of Cu at edges and corners.