Page 295 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 295
P1: GTV/GRI P2: GNH Final Pages
Encyclopedia of Physical Science and Technology EN010H-470 July 16, 2001 16:53
304 Nanosized Inorganic Clusters
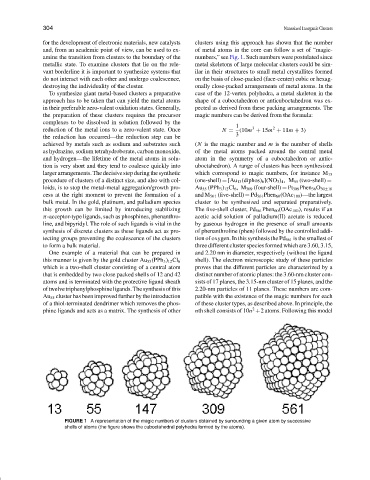
for the development of electronic materials, new catalysts clusters using this approach has shown that the number
and, from an academic point of view, can be used to ex- of metal atoms in the core can follow a set of “magic-
amine the transition from clusters to the boundary of the numbers,” see Fig. 1. Such numbers were postulated since
metallic state. To examine clusters that lie on the rele- metal skeletons of large molecular clusters could be sim-
vant borderline it is important to synthesize systems that ilar in their structures to small metal crystallites formed
do not interact with each other and undergo coalescence, on the basis of close-packed (face-center) cubic or hexag-
destroying the individuality of the cluster. onally close-packed arrangements of metal atoms. In the
To synthesize giant metal-based clusters a preparative case of the 12-vertex polyhedra, a metal skeleton in the
approach has to be taken that can yield the metal atoms shape of a cuboctahedron or anticuboctahedron was ex-
in their preferable zero-valent oxidation states. Generally, pected as derived from these packing arrangements. The
the preparation of these clusters requires the precursor magic numbers can be derived from the formula:
complexes to be dissolved in solution followed by the
1
2
3
reduction of the metal ions to a zero-valent state. Once N = (10m + 15m + 11m + 3)
the reduction has occurred—the reduction step can be 3
achieved by metals such as sodium and substrates such (N is the magic number and m is the number of shells
as hydrazine, sodium tetrahydroborate, carbon monoxide, of the metal atoms packed around the central metal
and hydrogen—the lifetime of the metal atoms in solu- atom in the symmetry of a cuboctahedron or antic-
tion is very short and they tend to coalesce quickly into uboctahedron). A range of clusters has been synthesized
largerarrangements.Thedecisivestepduringthesynthetic which correspond to magic numbers, for instance M 13
procedure of clusters of a distinct size, and also with col- (one-shell) = [Au 13 (diphos) 6 ](NO 3 ) 4 ,M 55 (two-shell) =
loids, is to stop the metal-metal aggregation/growth pro- Au 55 (PPh 3 ) 12 Cl 6 , M 309 (four-shell) = Pt 309 Phen 36 O 30±10
cess at the right moment to prevent the formation of a and M 561 (five-shell) = Pd 561 Phen 60 (OAc 180 )—the largest
bulk metal. In the gold, platinum, and palladium species cluster to be synthesized and separated preparatively.
this growth can be limited by introducing stabilizing The five-shell cluster, Pd 561 Phen 60 (OAc 180 ), results if an
π-acceptor-type ligands, such as phosphines, phenanthro- acetic acid solution of palladium(II) acetate is reduced
line, and bipyridyl. The role of such ligands is vital in the by gaseous hydrogen in the presence of small amounts
synthesis of discrete clusters as these ligands act as pro- of phenanthroline (phen) followed by the controlled addi-
tecting groups preventing the coalescence of the clusters tion of oxygen. In this synthesis the Pd 561 is the smallest of
to form a bulk material. three different cluster species formed which are 3.60, 3.15,
One example of a material that can be prepared in and 2.20 nm in diameter, respectively (without the ligand
shell). The electron microscopic study of these particles
this manner is given by the gold cluster Au 55 (PPh 3 ) 12 Cl 6
which is a two-shell cluster consisting of a central atom proves that the different particles are characterized by a
that is embedded by two close packed shells of 12 and 42 distinct number of atomic planes: the 3.60-nm cluster con-
atoms and is terminated with the protective ligand sheath sists of 17 planes, the 3.15-nm cluster of 15 planes, and the
oftwelvetriphenylphosphineligands.Thesynthesisofthis 2.20-nm particles of 11 planes. These numbers are com-
Au 55 cluster has been improved further by the introduction patible with the existence of the magic numbers for each
of a thiol-terminated dendrimer which removes the phos- of these cluster types, as described above. In principle, the
2
phine ligands and acts as a matrix. The synthesis of other nth shell consists of 10n +2 atoms. Following this model
FIGURE 1 A representation of the magic numbers of clusters obtained by surrounding a given atom by successive
shells of atoms (the figure shows the cuboctahedral polyhedra formed by the atoms).